|
ChemicalBook Optimization Suppliers |
| 名前: |
BEST-REAGENT |
| 電話番号: |
400-1166-196 18981987031 |
| 電子メール: |
cdhxsj@163.com |
|
| | 6-メチルキノキサリン-2,3-ジチオール2,3-カルボナート 製品概要 |
| 化学名: | 6-メチルキノキサリン-2,3-ジチオール2,3-カルボナート | | 英語化学名: | Chinomethionate | | 别名: | 1,3-Dithiolo[4,5-b]quinoxalin-2-one, 6-methyl-;3-quinoxalinedithiol,6-methyl-cycliccarbonate;3-quinoxalinedithiol,6-methyl-cyclicdithiocarbonate(ester);5-b)quinoxalin-2-one,6-methyl-3-dithiolo(4;5-b)quinoxalin-2-one,6-methyl-dithiolo(;6-methyl-1,3-dithiolo(4,5-b)quinoxalin-2-one;6-Methyl-1,3-Dithiolo[4,5-b]quinoxalin-2-one;6-methyl-2,3-quinoxalinedithiocarbonate | | CAS番号: | 2439-01-2 | | 分子式: | C10H6N2OS2 | | 分子量: | 234.3 | | EINECS: | 219-455-3 | | カテゴリ情報: | Q;Alphabetic;Analytical Standards | | Mol File: | 2439-01-2.mol | 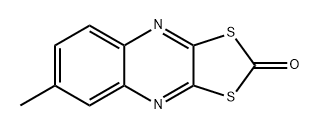 |
| | 6-メチルキノキサリン-2,3-ジチオール2,3-カルボナート 物理性質 |
| 融点 | 172° | | 沸点 | 476.6±55.0 °C(Predicted) | | 比重(密度) | 1.4147 (rough estimate) | | 蒸気圧 | 2.6 x 10-5 Pa (20 °C) | | 屈折率 | 1.6800 (estimate) | | 貯蔵温度 | 0-6°C | | 溶解性 | Chloroform (Slightly), Methanol (Slightly, Heated) | | 外見 | Solid | | 水溶解度 | 1 mg l-1 (20 °C) | | 酸解離定数(Pka) | -5.28±0.20(Predicted) | | 色 | Yellow crystals | | Merck | 13,7048 | | LogP | 3.78 at 20℃ | | NISTの化学物質情報 | Quinomethionate(2439-01-2) | | EPAの化学物質情報 | Chinomethionate (2439-01-2) |
| | 6-メチルキノキサリン-2,3-ジチオール2,3-カルボナート Usage And Synthesis |
| 外観 | 白色~うすい黄色, 結晶~粉末 | | 溶解性 | 水に不溶 (1mg/l at 20℃)。トルエン20g/l, ジクロロメタン40g/l, ヘキサン1.8g/l, イソプロパノール0.9g/l, シクロヘキサノン18g/l, ジメチルホルムアミド10g/l at 20℃。トルエンにやや溶けにくく、ヘキサンに溶けにくい。 | | 農薬用途 | 殺虫剤、殺菌剤、ダニ駆除剤 | | 説明 | Chinomethionate is a quinoxaline fungicide and acaricide introduced in 1968 to control powdery mildew and spider mites on fruits, ornamentals, cucurbits, cotton, coffee, tea, tobacco, walnuts, vegetables and glasshouse crops. It is nonsystemic, with contact activity only. | | 化学的特性 | Chinomethionate is a tan to yellow crystals. Soluble in benzene, toluene, and dioxane; insoluble in water. It has been shown to have low toxicity to mammals, birds and bees, but is highly toxic to fish and some aquatic invertebrates. | | 使用 | Chinomethionat exhibits both acaricidal and fungicidal activities.
It is a selective, non-systemic contact fungicide with both protective and
eradicant control of powdery mildews on fruits, cucurbits, vegetables,
ornamentals, tobacco and glasshouse crops. | | 定義 | ChEBI: A dithioloquinoxaline that results from the formal condensation of 6-methylquinoxaline-2,3-dithiol with phosgene. It has been used as a fungicide and acaricide for the control of mites and powdery mildew on citrus, vegetables, and walnuts, but is not appro
ed for use in the EU. | | 一般的な説明 | Yellow crystals. Non-corrosive. Used as a selective fungicide. | | 空気と水の反応 | Hydrolyzed in alkaline solution. | | 反応プロフィール | A member of the quinoxaline, dithiolane family. | | 危険性 | Toxic by ingestion and inhalation. | | 燃焼性と爆発性 | Not classified | | 安全性プロファイル | Moderately toxic by intraperitoneal, ingestion, and skin contact routes. A pesticide. When heated to decomposition it emits very toxic fumes of NOx and SOx. | | 環境運命予測 | Chemical/Physical. Reacts with ammonia forming 6-methyl-2,3-quinoxalinedithiol. | | 代謝経路 | Limited information is available to describe the degradation and metabolic
fate of chinomethionat. Hydrolytic cleavage of the dithiocarbonate
ring is the primary degradation reaction in water, plants and animals. | | Mode of action | Chinomethionate reacts with sulfur-containing amino acids in proteins, thereby disrupting the function of many enzymes and other proteins. Because there is no single target site, target site resistance is unlikely. | | Degradation | Chinomethionat was degraded at pH 4,7 and 9 at 22 °C with DT50 values
of 10 days, 3.3 days and 3.6 hours, respectively. It is degraded under
alkaline conditions to yield 6-methyl-2,3-quinoxalinedithiol (2) (PM) as
shown in Scheme 1. Clark and Loeffler (1980) reported the extensive
photodegradation of chinomethionat in benzene in a nitrogen atmosphere
under UV light to yield dimeric condensation products and benzene
insertion products. |
|