| Company Name: |
ExSyn Corp
|
| Tel: |
+91-2240546474 |
| Email: |
shailesh@exsyncorp.com |
| Products Intro: |
Product Name:Lenalidomide hydrochloride
CAS:1243329-97-6
Purity:98% Package:1 kg,5 kg, 10 kg,25kg And 1MT
|
| Company Name: |
LETOPHARM LIMITED
|
| Tel: |
+86-21-5821 5861 |
| Email: |
sales@letopharm.com |
| Products Intro: |
Product Name:LenalidoMide (hydrochloride)
CAS:1243329-97-6
|
| Company Name: |
SPIRO PHARMA
|
| Tel: |
|
| Email: |
eric_feng1954@126.com |
| Products Intro: |
Product Name:LenalidoMide (hydrochloride)
CAS:1243329-97-6
Purity:95% -98%HPLC Package:1GR;10GR;50GR;100GR;250GR;500GR;1KG;5KG;10KG;100KG
|
CC 5013 hydrochloride manufacturers
|
| | CC 5013 hydrochloride Basic information |
| Product Name: | CC 5013 hydrochloride | | Synonyms: | CC 5013 hydrochloride;CC5013 hydrochloride;CC-5013 hydrochloride;LenalidoMide (hydrochloride);RevliMid hydrochloride;Lenalidomide HCl | | CAS: | 1243329-97-6 | | MF: | C13H14ClN3O3 | | MW: | 295.72 | | EINECS: | | | Product Categories: | | | Mol File: | 1243329-97-6.mol | 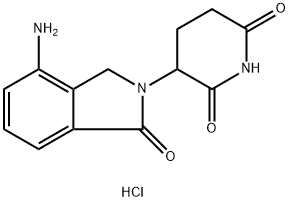 |
| | CC 5013 hydrochloride Chemical Properties |
| storage temp. | Store at -20°C | | solubility | insoluble in H2O; ≥1.83 mg/mL in EtOH; ≥72.2 mg/mL in DMSO | | form | solid |
| | CC 5013 hydrochloride Usage And Synthesis |
| Uses | Lenalidomide hydrochloride (CC-5013 hydrochloride), a derivative of Thalidomide, acts as molecular glue. Lenalidomide hydrochloride is an orally active immunomodulator. Lenalidomide hydrochloride (CC-5013 hydrochloride) is a ligand of ubiquitin E3 ligase cereblon (CRBN), and it causes selective ubiquitination and degradation of two lymphoid transcription factors, IKZF1 and IKZF3, by the CRBN-CRL4 ubiquitin ligase. Lenalidomide hydrochloride (CC-5013 hydrochloride) specifically inhibits growth of mature B-cell lymphomas, including multiple myeloma, and induces IL-2 release from T cells[1][2]. | | Biological Activity | lenalidomideis a derivative of thalidomide introduced in 2004. lenalidomide (revlimid, cc-5013) is a tnf-α secretion inhibitor with ic50 of 13 nm. | | in vitro | lenalidomide strongly induces il-2 and sil-2r production. lenalidomide-induced tyrosine phosphorylation of cd28 on t cells is followed by a down-stream activation of nf-κb [2]. lenalidomide and pomalidomide inhibits autoubiquitination of crbn in hek293 t cells expressing thalidomide-binding competent wild-type crbn, but not thalidomide-binding defective crbn (yw/aa). overexpression of crbn wild-type protein, but not crbn (yw/aa) mutant protein, in kms12 myeloma cells, amplifies pomalidomide-mediated reductions in c-myc and irf4 expression and increases in p21(waf-1) expression. long-term selection for lenalidomide resistance in h929 myeloma cell lines is accompanied by a reduction in crbn, while in df15r myeloma cells resistant to both pomalidomide and lenalidomide, crbn protein is undetectable [3]. | | in vivo | pharmacokinetic studies evaluated doses of 0.5, 1.5, 5, and 10 mg/kg iv and 0.5 and 10 mg/kg doses for ip and oral routes. liquid chromatography-tandem mass spectrometry was used to quantify lenalidomide in plasma, brain, lung, liver, heart, kidney, spleen, and muscle [4]. treatment with either thalidomide or lenalidomide attenuated weight loss, enhanced motor performance, decreased motor neuron cell death, and significantly increased the life span in g93a transgenic mice [5]. | | IC 50 | value: 13 nm [1] lenalidomideis a derivative of thalidomide introduced in 2004. lenalidomide (revlimid, cc-5013) is a tnf-α secretion inhibitor with ic50 of 13 nm. in vitro: lenalidomide strongly induces il-2 and sil-2r production. lenalidomide-induced tyrosine phosphorylation of cd28 on t cells is followed by a down-stream activation of nf-κb [2]. lenalidomide and pomalidomide inhibits autoubiquitination of crbn in hek293 t cells expressing thalidomide-binding competent wild-type crbn, but not thalidomide-binding defective crbn (yw/aa). overexpression of crbn wild-type protein, but not crbn (yw/aa) mutant protein, in kms12 myeloma cells, amplifies pomalidomide-mediated reductions in c-myc and irf4 expression and increases in p21(waf-1) expression. long-term selection for lenalidomide resistance in h929 myeloma cell lines is accompanied by a reduction in crbn, while in df15r myeloma cells resistant to both pomalidomide and lenalidomide, crbn protein is undetectable [3]. in vivo: pharmacokinetic studies evaluated doses of 0.5, 1.5, 5, and 10 mg/kg iv and 0.5 and 10 mg/kg doses for ip and oral routes. liquid chromatography-tandem mass spectrometry was used to quantify lenalidomide in plasma, brain, lung, liver, heart, kidney, spleen, and muscle [4]. treatment with either thalidomide or lenalidomide attenuated weight loss, enhanced motor performance, decreased motor neuron cell death, and significantly increased the life span in g93a transgenic mice [5]. toxicity: international staging system iii received a combination therapy of lenalidomide (15 mg, day 1 - 21) with dexamethasone (40 mg, day 1, 8, 15, 22). after 4 days on chemotherapy, he experienced worsened dyspnea and was urgently hospitalized because of acute respiratory failure [6]. | | References | [1] Omran A, et al. Effects of MRP8, LPS, and lenalidomide on the expressions of TNF-α , brain-enriched, and inflammation-related microRNAs in the primary astrocyte culture. ScientificWorldJournal. 2013 Sep 21;2013:208309. DOI:10.1155/2013/208309
[2] Kr?nke J, et al. Lenalidomide induces degradation of IKZF1 and IKZF3. Oncoimmunology. 2014 Jul 3;3(7):e941742. DOI:10.4161/21624011.2014.941742
[3] Minzel W, et al. Small Molecules Co-targeting CKIα and the Transcriptional Kinases CDK7/9 Control AML in Preclinical Models. Cell. 2018 Sep 20;175(1):171-185.e25. DOI:10.1016/j.cell.2018.07.045
[4] Kotla V, et al. Mechanism of action of lenalidomide in hematological malignancies. J Hematol Oncol. 2009 Aug 12;2:36. DOI:10.1186/1756-8722-2-36
[5] Lopez-Girona A, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012 Nov;26(11):2326-35. DOI:10.1038/leu.2012.119
[6] Rozewski DM, et al. Pharmacokinetics and tissue disposition of lenalidomide in mice. AAPS J. 2012 Dec;14(4):872-82. DOI:10.1208/s12248-012-9401-2 |
| | CC 5013 hydrochloride Preparation Products And Raw materials |
|