|
ChemicalBook Optimization Suppliers |
|
| 融点 | 65-67 °C (lit.) | | 沸点 | 297-298 °C (lit.) | | 比重(密度) | 1.0120 (rough estimate) | | 蒸気圧 | 0.000076 hPa (20 °C) | | 屈折率 | 1.5727 (estimate) | | 闪点 | 160 °C | | 貯蔵温度 | Store below +30°C. | | 溶解性 | 0.52g/l insoluble | | 外見 | Crystalline Solid | | 酸解離定数(Pka) | 13.55±0.20(Predicted) | | 色 | White to beige | | 臭い (Odor) | at 100.00 %. weedy green rose | | においのタイプ | green | | 水溶解度 | Slightly soluble in water. | | Merck | 14,1090 | | BRN | 1424379 | | 安定性: | Stable. Combustible. Incompatible with strong oxidizing agents, acid chlorides, acid anhydrides, acids. | | LogP | 2.670 | | CAS データベース | 91-01-0(CAS DataBase Reference) | | NISTの化学物質情報 | Benzenemethanol, «alpha»-phenyl-(91-01-0) | | EPAの化学物質情報 | Benzenemethanol, .alpha.-phenyl- (91-01-0) |
| 主な危険性 | Xi | | Rフレーズ | 36/37/38 | | Sフレーズ | 26-36-24/25 | | WGK Germany | 2 | | RTECS 番号 | DC7452000 | | Hazard Note | Irritant | | TSCA | Yes | | HSコード | 29062900 | | 毒性 | LD50 orally in Rabbit: 5000 mg/kg LD50 dermal Rabbit > 5000 mg/kg |
| | ベンズヒドロール Usage And Synthesis |
| 外観 | 白色〜わずかにうすい黄色, 結晶性粉末〜粉末又は塊又はフレーク | | 溶解性 | エタノールに溶ける。エーテル、酢酸、クロロホルム、四塩化炭素、二硫化炭素に溶け易い。冷リグロインにほとんど溶けない。 | | 解説 | ベンズヒドロール,結晶.融点68 ℃,沸点180 ℃(2.66 kPa).エタノール,エーテル,クロロホルムなどに可溶,水に難溶.加熱するとジ(ベンズヒドリル)エーテルを生じ,還元するとジフェニルメタンとなる.有機合成原料に用いられる. 森北出版「化学辞典(第2版)
| | 用途 | ジフェニルメタノール(diphenylmethanol)は、香料や薬剤の製造に使われる。 | | 用途 | 有機合成原料。 | | 製造 | ベンズヒドロール,ジフェニルメタノールともいう.ベンゾフェノンを亜鉛末とアルカリで還元するか,フェニルマグネシウムハライドとベンズアルデヒドとのグリニャール反応によってつくられる. | | 化学的特性 | off-white powder | | 使用 | Anchoring of carboxylic acids and alcohols | | 使用 |
- Benzhydrols are industrially important compounds. On oxidation Benzhydrols yields Benzophenone, which are useful synthones for fullerenes, bioactive oxygen heterocycles, dyes and medicines. Various Cr (VI) and other oxidizing agents are used for oxidizing benzhydrol.
- Benzhydrol is widely used as intermediates in pharmaceuticals (including antihistamines), agrochemicals, perfumes and other organic compounds. It is used as a fixative in the perfume industry. It is involved in polymerization reaction as a terminating group. It is used as precursor to prepare modafinil, benztropine and diphehydramine.
- Intermediate in the preparation of Modafinil (M482500).
| | 定義 | ChEBI: Diphenylmethanol is a secondary alcohol that is diphenylmethane which carries a hydroxy group at position 1. It has a role as a rat metabolite, a bacterial xenobiotic metabolite, a human xenobiotic metabolite and a human urinary metabolite. It is a secondary alcohol and a member of benzyl alcohols. It derives from a hydride of a diphenylmethane. | | 反応性 | Benzhydrol is oxidized to benzophenone, by sodium hypochlorite (commonly known as bleach) in the presence of a phase-transfer catalyst.
Synthesis: In a 20-mL green capped vial, place 1.5 mL of ethyl acetate, 100 mg (0.54 mmol) of benzhydrol and a few drops of methyltricaprylammonium chloride solution (Stark's catalyst or tricaprylmethylammonium chloride). Add a half-inch magnetic stirring bar, and stir until all reagents are dissolved. Cool the solution in an ice bath and add 2 mL of 5% NaOCl (aq) (bleach) dropwise using a 2.5- mL syringe. After the addition of the hypochlorite is complete, allow the reaction to stir for five minutes in the ice-water bath and then stir for a period of one hour at room temperature.
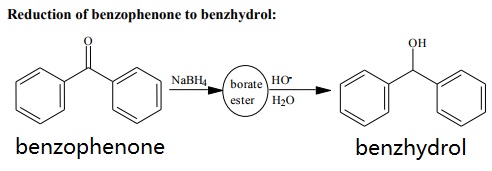
Reduction of benzophenone to benzhydrol.
4MPDC (4-Methyl pyridinium di chromate) is used as oxidizing agent to oxidize benzhydrol. PDC is a mild and selective oxidizing agent and is soluble in water and many organic solvents. Therefore, advantage over inorganic dichromate. | | Synthesis Reference(s) | Canadian Journal of Chemistry, 50, p. 3058, 1972 DOI: 10.1139/v72-485
Journal of the American Chemical Society, 55, p. 391, 1933 DOI: 10.1021/ja01328a057
Tetrahedron Letters, 29, p. 139, 1988 DOI: 10.1016/S0040-4039(00)80036-2 | | 一般的な説明 | Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards | | 純化方法 | Crystallise benzhydrol from hot H2O or pet ether (b 60-70o), pet ether containing a little *benzene, from CCl4, or EtOH (1mL/g). An additional purification step includes passage of a *benzene solution through an activated alumina column. It sublimes in a vacuum. Also recrystallise it three times from MeOH/H2O [Naguib J Am Chem Soc 108 128 1986]. [Beilstein 6 IV 4648.] |
|