|
ChemicalBook Optimization Suppliers |
| 名前: |
Energy Chemical |
| 電話番号: |
021-021-58432009 400-005-6266 |
| 電子メール: |
sales8178@energy-chemical.com |
|
| 化学名: | ベンジルイソシアニド | | 英語化学名: | Benzyl isocyanide | | 别名: | HANSA ISN-0155;BENZYL ISOCYANIDE;BIO-FARMA BF000644;Benzyl carbylamine;Phenylmethyl isocyanide;Benzyl isocyanide,98%;Benzyl lsocyanide;Benzyl isocyanide, 98% 1GR | | CAS番号: | 10340-91-7 | | 分子式: | C8H7N | | 分子量: | 117.15 | | EINECS: | 233-738-9 | | カテゴリ情報: | Isocyanides;Nitrogen Compounds;Organic Building Blocks | | Mol File: | 10340-91-7.mol | 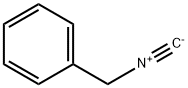 |
| 沸点 | 105-106 °C75 mm Hg(lit.) | | 比重(密度) | 0.962 g/mL at 25 °C(lit.) | | 屈折率 | n20/D 1.521(lit.) | | 闪点 | 175 °F | | 貯蔵温度 | -20°C | | 酸解離定数(Pka) | 27.4 | | 外見 | clear liquid | | 色 | Colorless to Red to Green |
| | ベンジルイソシアニド Usage And Synthesis |
| 外観 | 無色~赤色~緑色透明液体 | | 化学的特性 | clear colorless to yellowish liquid | | 使用 | Benzyl Isocyanide is used in the preparation of arylamide compounds. also used as a catalyst in the cycloaddition of norbornenes with internal and terminal acetylenes. | | 主な応用 | Benzyl isocyanide forms phosphaalkene-containing complexes. Benzyl isocyanide was used in the synthesis of Ru(II) complexes containing hydrazine and benzyl isocyanide ligands. It was used in a three-component coupling process leading to O- and N-arylamides. | | 合成 | Synthesis of benzyl isocyanide
In a 500-mL, round-bottomed flask equipped with a magnetic stirring bar and a pressure-equalizing funnel are placed 5-benzylaminotetrazole (10.5 g, 60 mmol), 100 mL of 10% sodium hydroxide solution, and 70 mL of dichloromethane. The mixture is cooled to 0°C and a solution of NaOBr in water (165 mL, 65 mmol) (Note 2) is added with vigorous stirring over a 15-min period (Note 3). The dichloromethane layer is separated and the aqueous phase extracted with five 50-mL portions of dichloromethane. The combined dichloromethane extracts are dried over anhydrous MgSO4, the drying agent is removed by filtration, and the dichloromethane is removed by simple distillation. The pressure is then reduced to ~20 mm with an aspirator and benzyl isocyanide is distilled at 98–100°C; yield: 5.91 g (84%) (Note 4) and (Note 5).
Organic Syntheses, Coll. Vol. 7, p. 27, 1990
http://www.orgsyn.org |
|