|
ChemicalBook Optimization Suppliers |
| 名前: |
Alfa Aesar |
| 電話番号: |
400-6106006 |
| 電子メール: |
saleschina@alfa-asia.com |
| 名前: |
Energy Chemical |
| 電話番号: |
021-021-58432009 400-005-6266 |
| 電子メール: |
sales8178@energy-chemical.com |
|
| 化学名: | 2,6-ジメチル-4-ヘプタノン | | 英語化学名: | 2,6-Dimethyl-4-heptanone | | 别名: | 2,6-DIMETHYL-4-HEPTANONE;2,6-Dimethyl-4-heptanone, remainder mainly 4,6-dimethyl-2-heptanone;Diisobutyl ketone~Isovalerone;Dimethylheptanone;2 6-DIMETHYL-4-HEPTANONE 99+%;2,6-DIMETHYL-4-HEPTANONE, TECH., 80%;2,6-Dimethyl-4-Heptanone,Purified;2,6-Dimethyl-4-heptanone, remainder mainly 4,6-dimethyl-2-heptanone, tech., 80% | | CAS番号: | 108-83-8 | | 分子式: | C9H18O | | 分子量: | 142.24 | | EINECS: | 203-620-1 | | カテゴリ情報: | | | Mol File: | 108-83-8.mol | 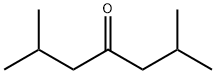 |
| 融点 | -46 °C | | 沸点 | 165-170 °C(lit.) | | 比重(密度) | 0.808 g/mL at 25 °C(lit.) | | 蒸気密度 | 4.9 (vs air) | | 蒸気圧 | 1.7 mm Hg ( 20 °C) | | 屈折率 | n20/D 1.412(lit.) | | FEMA | 3537 | 2,6-DIMETHYL-4-HEPTANONE | | 闪点 | 120 °F | | 貯蔵温度 | Store below +30°C. | | 溶解性 | Miscible with ethanol, ether, carbon tetrachloride, chloroform, benzene and most organic liquids. | | 外見 | Liquid | | 比重 | 0.810 (20/4℃) | | 色 | Clear colorless to slightly yellow | | 臭い (Odor) | Mild; characteristic ketonic. | | 爆発限界(explosive limit) | 0.8-6.2%, 100°F | | においのタイプ | green | | 水溶解度 | 0.05 g/100 mL | | JECFA Number | 302 | | BRN | 1743163 | | Henry's Law Constant | 6.36(x 10-4 atm?m3/mol) at 20 °C (approximate - calculated from water solubility and vapor pressure) | | 暴露限界値 | TLV-TWA 150 mg/m3 (25 ppm); IDLH
1000 ppm. | | Dielectric constant | 9.9100000000000001 | | 安定性: | Stable. Flammable. Incompatible with strong oxidizing agents. | | LogP | 3.71 at 20℃ | | CAS データベース | 108-83-8(CAS DataBase Reference) | | NISTの化学物質情報 | 4-Heptanone, 2,6-dimethyl-(108-83-8) | | EPAの化学物質情報 | Diisobutyl ketone (108-83-8) |
| 主な危険性 | Xi | | Rフレーズ | 10-37 | | Sフレーズ | 24 | | RIDADR | UN 1157 3/PG 3 | | WGK Germany | 1 | | RTECS 番号 | MJ5775000 | | 自然発火温度 | 745 °F | | TSCA | Yes | | 国連危険物分類 | 3 | | 容器等級 | III | | HSコード | 29141990 | | 有毒物質データの | 108-83-8(Hazardous Substances Data) | | 毒性 | LD50 orally in Rabbit: 5750 mg/kg LD50 dermal Rabbit 16000 mg/kg | | IDLA | 500 ppm |
| | 2,6-ジメチル-4-ヘプタノン Usage And Synthesis |
| 外観 | 無色〜わずかにうすい褐色, 澄明の液体 | | 溶解性 | 水に難溶, 各種有機溶剤に可溶。エタノール、アセトン及びクロロホルムに極めて溶けやすく、水に極めて溶けにくい。 | | 用途 | 原子吸光分析における抽出溶媒。 | | 用途 | 塗料用溶剤 | | 用途 | 有機合成原料。 | | 用途 | ニトロセルロース?ゴム?合成樹脂?塗料溶剤、化学工業日報社 | | 説明 | 2, 6-Dimethyl-4-heptanone, also known as diisobutyl ketone, belongs to the family of ketones, being a flavoring ingredient. It can also be used as the extraction solvent for the determination of ten trace metals (V, Cr, Fe, Co, Ni, Cu, Zn, Mo, Cd, Pb) in aqueous samples with plasma atomic emission spectrometry. Similar logic can also be applied to the measurement of phosphorus using 2, 6-dimethyl-4-heptanone as the extraction agent. It is also an important organic solvent widely used as industrial intermediates. | | 化学的特性 | 5-Methyl-3-heptanone is a colorless liquid with low solubility in water
(0.3 wt %); water is poorly soluble in the ketone
(0.9 wt %). 5-Methyl-3-heptanone is highly soluble in common organic solvents. | | 物理的性質 | Clear, colorless liquid with a mild, sweet, ether-like odor. Odor threshold concentration is 0.11
ppm (quoted, Amoore and Hautala, 1983). | | 天然物の起源 | Reported found in baked potato and wheaten bread. | | 使用 | 2,6-Dimethyl-4-heptanone is used as a coating solvent. It is an active component of mint oil. It acts as a dispersant for organosol type resins. It is involved in the antigerminative treatment of bulbs and tubers. Further, it is used as a solvent for nitrocellulose. In addition to this, it acts as an intermediate in the preparation of inhibitors, active pharmaceutical ingredients and dyes. | | 使用 | Diisobutyl ketone is used as a solvent fornitrocellulose, lacquers, and synthetic resins;in organic syntheses. | | 使用 | Diisobutyl Ketone is a component of mint oil and L-carvone solutions for fungicidal and antigerminative treatment of bulbs and tubers. | | 使用 | Diisobutyl ketone (DIBK) is a transparent liquid with a distinct odor and a high boiling point. It is an heavy-end byproduct of producing MIBK. DIBK is used in many applications such as nitrocellulose lacquers, synthetic resins, coatings and stains, paint strippers, leather finishings, adhesives, printing and coating inks, cleaning and dregreasing, Flavors and fragrances, solvent and re-crystallization aid for pharmaceuticals, mining, and as a chemical intermediate.
DIBK has good activity for many synthetic resins including nitrocellulose, rosin esters, phenolics, hydrocarbons, alkyds, polyesters, and acrylics. It is useful as a retarder solvent to improve flow and minimize humidity blushing. The low density and low surface tension of DIBK enables formulators to develop high-solids coatings with low VOC content and excellent flow and leveling properties.
DIBK has excellent viscosity reduction for and reduces surface tension in high solid’s coatings. It has good volume-to-weight advantage over other classes of solvents used in coatings. It is a non-HAP (Hazardous Air Pollutant) solvent. | | 定義 | ChEBI: 2-Methyl-4-heptanone is a ketone. | | 調製方法 | Diisobutyl ketone is produced by hydrogenation of phorone
or by metal-catalyzed decomposition of isovaleric acid.It is also a by-product in the manufacture of methyl isobutyl
ketone. | | Synthesis Reference(s) | Journal of the American Chemical Society, 95, p. 6876, 1973 DOI: 10.1021/ja00801a081 | | 一般的な説明 | A clear colorless liquid. Flash point 140°F. Less dense than water and insoluble in water. Vapors heavier than air. | | 空気と水の反応 | Flammable. Insoluble in water. | | 反応プロフィール | 2,6-Dimethyl-4-heptanone may attack some plastics. 2,6-Dimethyl-4-heptanone reacts with oxidizers. | | 健康ハザード | Inhalation of vapor causes irritation of nose and throat. Ingestion causes irritation of mouth and stomach. Vaporirritates eyes. Contact with liquid irritates skin. | | 健康ハザード | Inhalation of the vapors of diisobutyl ketonecan produce irritation of the eyes, nose, andthroat. At 25 ppm its odor was unpleasant, but the irritation effect on humanswas insignificant. At 50 ppm the irritationwas mild. A 7- hour exposure to 125 ppmhad no adverse effect on rats; however, at250 ppm, female rats developed increasedliver and kidney weights. An 8-hour expo�sure to 2000 ppm was lethal. Ingestion ofthis compound can cause the symptoms ofheadache, dizziness, and dermatitis.
LD50 value, oral (rats): 5.8 g/kg. | | 燃焼性と爆発性 | Not classified | | 化学反応性 | Reactivity with Water No reaction; Reactivity with Common Materials: May attack some forms of plastics; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent. | | 安全性プロファイル | Moderately toxic by
ingestion and inhalation. Mddly toxic by skin
contact. Human systemic effects by
inhalation: headache, nausea or vomiting,
and unspecified eye effects. An eye and skin
irritant. Narcotic in high concentrations.
Flammable liquid when exposed to heat or
flame; can react with oxidizing materials. To
fight fire, use Con, dry chemical, water
spray, mist or fog. When heated to
decomposition it emits acrid smoke and
fumes. See also KETONES. | | 職業ばく露 | Human Data;Primary Irritant. Diisobutyl ketone is used as a solvent; as a dispersant for resins; and as an intermediate in the synthesisof pharmaceuticals and pesticides. | | 応急処置 | If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the | | 環境運命予測 | Biological. Using the BOD technique to measure biodegradation, the mean 5-d BOD value (mM
BOD/mM diisobutyl ketone) and ThOD were 4.86 and 37.4%, respectively (Vaishnav et al.,
1987).
Chemical/Physical. Diisobutyl ketone will not hydrolyze because it has no hydrolyzable
functional group.
At an influent concentration of 300 mg/L, treatment with GAC resulted in nondetectable
concentrations in the effluent. The adsorbability of the carbon used was 60 mg/g carbon (Guisti et
al., 1974). | | 貯蔵 | Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials. Prior to working with DIBK you should be trained on itsproper handling and storage. Before entering confined spacewhere DIBK may be present, check to make sure that anexplosive concentration does not exist. Store in tightlyclosed containers in a cool, well-ventilated area. Metal containers involving the transfer of this chemical should begrounded and bonded. Where possible, automatically pumpliquid from drums or other storage containers to processcontainers. Drums must be equipped with self-closingvalves, pressure vacuum bungs, and flame arresters. Useonly nonsparking tools and equipment, especially whenopening and closing containers of this chemical. Sources ofignition, such as smoking and open flames, are prohibitedwhere this chemical is used, handled, or stored in a mannerthat could create a potential fire or explosion hazard.Wherever this chemical is used, handled, manufactured, orstored, use explosion-proof electrical equipment andfittings. | | 輸送方法 | This compound requires a shipping label of“FLAMMABLE LIQUID.” It falls in Hazard Class 3 andPacking Group II. | | 不和合性 | Forms explosive mixture with air.Incompatible with strong acids, aliphatic amines, strongoxidizers. Attacks some forms of plastics, coatings, andrubber. | | 廃棄物の処理 | Incineration, molten metal salt destruction. | | 参考文献 | Bone, K. M., and W. D. Hibbert. "Solvent extraction with ammonium pyrrolidinedithiocarbamate and 2,6-dimethyl-4-heptanone for the determination of trace metals in effluents and natural waters." Analytica Chimica Acta 107.JUN(1979):219-229.
Miyazaki, Akira, A. Kimura, and Y. Umezaki. "Determination of ng ml-1 levels of phosphorus in waters by diisobutyl ketone extraction and inductively coupled plasma atomic emission spectrometry." Analytica Chimica Acta 127.96(1981):93-101.
Zhang, Fagen, et al. "Comparative metabolism and pharmacokinetics of diisobutyl ketone and diisobutyl carbinol in male SD rats." Toxicology Letters 232.1(2015):175-181. |
|