|
ChemicalBook Optimization Suppliers |
|
| 化学名: | アクチノマイシンD | | 英語化学名: | Actinomycin D | | 别名: | 2-Amino-N,N'-bis[hexadecahydro-2,5,9-trimethyl-6,13-bis(1-methylethyl)-1,4,7,11,14-pentaoxo-1H-pyrrolo[2,1-i][1,4,7,10,13]oxatetraazacyclohexadecin-10-yl]-4,6-dimethyl-3-oxo-3H-phenoxazine-1,9-dicarboxamide;Actinomycin D, Actinomycin IV, Actinomycin C1, Dactinomycin;Dactinomycin, DTM;Actinomycin C1, Dactinomycin;Actactinomycin A IV;Cosmogen;actinomycin7;actinomycinaiv | | CAS番号: | 50-76-0 | | 分子式: | C62H86N12O16 | | 分子量: | 1255.42 | | EINECS: | 200-063-6 | | カテゴリ情報: | Caspases/Apoptosis;Antibiotics;Intermediates & Fine Chemicals;Peptides;Pharmaceuticals;Antibiotic Explorer;COSMEGEN;antibiotic | | Mol File: | 50-76-0.mol | 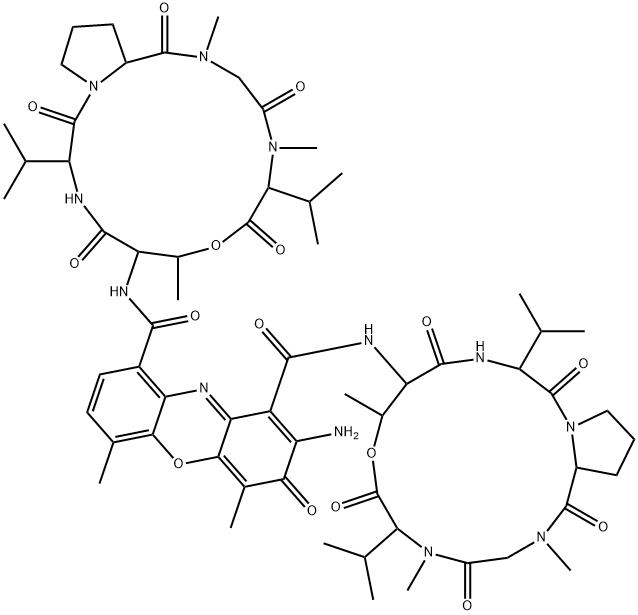 |
| 融点 | 251-253 °C | | 沸点 | 848°C (rough estimate) | | 比重(密度) | 1.0757 (rough estimate) | | 屈折率 | 1.5700 (estimate) | | 闪点 | 87℃ | | 貯蔵温度 | 2-8°C | | 溶解性 | ethanol, DMSO: Stable in aqueous solutions at 2-8 °C.soluble | | 酸解離定数(Pka) | 8.94±0.70(Predicted) | | 外見 | powder | | 色 | red, powder | | 光学活性 (optical activity) | [α]/D alpha: 28/D (Rotation: -315 degrees (c=0.25% in methanol)) | | 水溶解度 | SOLUBLE | | Merck | 13,2828 | | BRN | 605235 | | 安定性: | Stable, but light sensitive, especially in dilute solution. Incompatible with strong acids, strong bases, strong oxidizing agents. Combustible. | | CAS データベース | 50-76-0(CAS DataBase Reference) | | IARC | 3 (Vol. 10, Sup 7) 1987 | | EPAの化学物質情報 | Actinomycin D (50-76-0) |
| | アクチノマイシンD Usage And Synthesis |
| 外観 | 黄赤色, 結晶性粉末〜粉末 | | 解説 | アクチノマイシンD,橙赤(とうせき)色ないし赤色の結晶または結晶性粉末。ウィルムス腫瘍の特効薬で、絨毛(じゅうもう)上皮腫、破壊性胞状奇胎にも適応される。DNA(デオキシリボ核酸)のグアニンと結合して複合体をつくるところからDNA依存性のRNAポリメラーゼの作用が阻害され、RNA(リボ核酸)の生成が抑制されることが作用機序(メカニズム)と考えられている。注射剤として使用される。
小学館 日本大百科全書(ニッポニカ) )
| | 用途 | DNA に結合し、RNA 合成(特
に rRNA 合成)を選択的に阻害し、抗腫瘍作
用を示します。 | | 効能 | 抗悪性腫瘍薬 | | 製造 | アクチノマイシンDともいい、ストレプトミセス・パルブルスStreptomyces parvullusの産生する抗悪性腫瘍(しゅよう)性抗生物質である。 | | 商品名 | コスメゲン (ノーベルファーマ) | | 使用上の注意 | 不活性ガス封入 | | 説明 | Actinomycin D (50-76-0) is a cyclopeptide antibiotic and intercalating transcription inhibitor with anti-neoplastic activity. Potent inhibitor of RNA polymerase.1?Induces apoptosis in a variety of cancer cell lines2,3via the intrinsic pathway4.? Upregulates proapoptotic PUMA and downregulates Bcl-2 mRNA in peripheral blood lymphocytes.5 | | 化学的特性 | red crystalline powder | | 化学的特性 | Actinomycin D is a combustible, bright red crystalline solid. | | Originator | Cosmegen,Merck Sharp and
Dohme,US,1965 | | 使用 | An antineoplastic antibiotic that inhibits cell proliferation by forming a stable complex with DNA and blocking the movement of RNA polymerase which interferes with DNA-dependent RNA synthesis. Induces apoptosis. Potent antitumor agent. | | 使用 | Antibiotic substance belonging to the actinomycin complex, produced by several Streptomyces spp. Antineoplastic | | 使用 | antineoplastic, intercalating agent | | 使用 | Actinomycin D is the most studied member of a family of unique bicyclic depsipeptides produced by several Streptomyces species. The depsipeptides are linked by a heterocyclic benzoxazone "anchor" that gives the metabolites a highly distinctive red/orange colour. Actinomycin D exhibits potent antibiotic and antitumour activity via DNA intercalation leading to the inhibition of nucleic acid synthesis. Tumour cell death has been demonstrated to occur by apoptosis. | | 定義 | ChEBI: Actinomycin D is an actinomycin. It has a role as a mutagen. | | 適応症 | Dactinomycin (actinomycin D, Cosmegen) is one of a
family of chromopeptides produced by Streptomyces. It
is known to bind noncovalently to double-strand DNA
by partial intercalation, inhibiting DNA-directed RNA
synthesis. The drug is most toxic to proliferating cells,
but it is not specific for any one phase of the cell cycle.
Resistance to dactinomycin is caused by decreased ability
of tumor cells to take up and retain the drug, and it
is associated with cross-resistance to vinca alkaloids, the
anthracyclines, and certain other agents (multidrug resistance). | | Manufacturing Process | An incubated culture of Actinomyces antibioticus was prepared using a
medium consisting of 1% tryptone-peptone, 0.5% starch, 0.2% K2HPO4, 0.2%
NaCl and 0.25% agar in distilled water, grown at a temperature of
approximately 25° to 35°C, the incubation being complete after 6 to 10 days.
50 liters of this incubated culture are extracted approximately six times with
ether, using 20 liters of ether for each extraction.
The final extract is faintly pale yellow in color, whereas the previous extracts
are orange. The combined ether extracts are concentrated to dryness and
about 3 grams of a reddish-brown residue is obtained. The residue is stirred
with approximately 400 cc of petroleum ether for two to three hours, the
solvent decanted and the residue treated again with approximately 400 cc of
petroleum ether. A pale yellow oil constituting crude actinomycin B is
recovered by evaporation from the petroleum ether.
The dark petroleum ether insoluble residue is dissolved in 1 liter of benzene
with gentle heating. Usually a small amount of black amorphous material
remains undissolved and is filtered off. The benzene solution is permitted to
drop through a chromatographic tower (60 x 5 cm) packed with aluminum
oxide (according to Brockman). The pigment is readily adsorbed. The column
is washed with about 1 liter of benzene during which operation very little
migration of the color bands occurs.
The column is then washed with benzene-acetone solution (15:85) whereby a
chromatogram develops. By continued washing, light yellow colored pigments
pass out of the column. When the main band (orange-red) reaches the lower
end of the column, a solution of 30:70 acetone-benzene is passed through the
column. The latter solvent elutes the pigment and when the eluate is very
pale in color, washing is discontinued.
The eluate is concentrated to dryness under reduced pressure, taken up in 25
cc of hot acetone, filtered, and diluted with ether. The pigment which
crystallizes as red-brick colored platelets is essentially pure but may be
recrystallized if desired from hot ethyl acetate. An analysis of the product
showed C = 59.01; H = 6.81; N = 13.38. | | brand name | Cosmegen (Ovation). | | Therapeutic Function | Cancer chemotherapy | | 一般的な説明 | Dactinomycin is available in vials containing 0.5 mg of drugfor reconstitution in sterile water for IV administration Thisantibiotic is most effective in the treatment of rhabdomyosarcomaand Wilms tumor in children as well as in the treatmentof choriocarcinoma, Ewing sarcoma, Kaposi sarcoma, andtesticular carcinoma. The pharmacokinetics of dactinomycinhas not been well characterized, but it appears to concentratein nucleated blood cells. The agent is 5% to 15% plasmaprotein bound and is excreted mostly unchanged in urineand bile. Other metabolites have not been characterized.The terminal elimination half-life is 30 to 40 hours.Myelosuppression is dose limiting with both leucopenia andthrombocytopenia being the most likely presentation. Nauseaand vomiting occur shortly (2 hours) after treatment and maybe severe. Mucositis and diarrhea also result from irritation ofthe GI tract. Hair loss is commonly associated with the agentas is hyperpigmentation of the skin and erythema. | | 一般的な説明 | Bright red rhomboid prisms or red powder. | | 空気と水の反応 | Water soluble. | | 反応プロフィール | Actinomycin D can react with strong oxidizing agents, strong acids and strong bases. | | 火災危険 | Flash point data for Actinomycin D are not available. Actinomycin D is probably combustible. | | 生物活性 | Anti-neoplastic antibiotic. Inhibits RNA polymerase and is a potent inducer of apoptosis. | | 作用機序 | Dactinomycin is cleared rapidly from plasma, does
not enter the brain, is not appreciably metabolized or
protein bound, and is gradually excreted in both bile
and urine.Virtually no drug is detected in CSF. | | 臨床応用 | Dactinomycin is used in curative combined treatment
of Wilms’ tumor, Ewing’s sarcoma, rhabdomyosarcoma,
and gestational choriocarcinoma. It is active in
testicular tumors, lymphomas, melanomas, and sarcomas,
although its use in most of these malignancies has
been supplanted by other agents. | | 副作用 | The major side effects of dactinomycin are severe
nausea, vomiting, and myelosuppression. Mucositis, diarrhea,
alopecia, and radiation recall reactions may occur.
The drug is immunosuppressive and carcinogenic. | | 職業ばく露 | An antibiotic product from streptomyces, used as anticancer and veterinary drug | | Veterinary Drugs and Treatments | Dactinomycin has been used as adjunctive treatment of lymphoreticular
neoplasms,
bone and soft tissue sarcomas, and carcinomas
in small animals. It appears to have low efficacy against most carcinomas
and sarcomas. It is being investigated as a part of protocols
for rescue therapy for canine lymphomas. | | 薬物相互作用 | Potentially hazardous interactions with other drugs
Antipsychotics: increased risk of agranulocytosis
with clozapine - avoid.
Cytotoxics: increased risk of hepatotoxicity with
vincristine.
Vaccines: risk of generalised infections with live
vaccines - avoid. | | 代謝 | Intravenous doses of dactinomycin are rapidly distributed
with high concentrations in bone marrow and nucleated
cells. It undergoes only minimal metabolism and is
slowly excreted in urine and bile. 15% is eliminated by
hepatic metabolism. Approximately 30% of the dose was
recovered in the urine and faeces in 1 week. | | 貯蔵 | +4°C | | 輸送方法 | UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. | | 純化方法 | It crystallises as bright red rhombic crystals from absolute EtOH or from MeOH/EtOH (1:3). It will also crystallise from EtOAc/cyclohexane (m 246-247o dec), CHCl3/pet ether ( m 245-246o dec), and EtOAc/MeOH/*C6H6 (m 241-243o dec). Its solubility in MeCN is 1mg/mL. [] D varies from -296o to -327o (c 0.2, MeOH). max (MeOH) 445, 240nm (log � 4.43, 4.49), max (MeOH, 10N HCl, 1:1) 477nm (log � 4.21) and max (MeOH, 0.1N NaOH) 458, 344, 285 (log � 3.05, 4.28, 4.13). It is HIGHLY TOXIC, light sensitive and anti-neoplastic. [Bullock & Johnson, J Chem Soc 3280 1957, Beilstein 27 III/IV 9642.] | | 不和合性 | Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. | | 参考文献 | 1) Wagner?et al.(2013)?RNA Polymerase II acts as an RNA-dependent RNA polymerase to extend and destabilize a non-coding RNA ; EMBO J.?32?781
2) J. Kleeff?et al.?(2000)?Actinomycin D induces apoptosis and inhibits growth of pancreatic cancer cells; Int. J. Cancer,?86?399
3) Kasim?et al.?(2013)?Live fluorescence and transmission-through-dye microscopic study of actinomycin D-induced apoptosis and apoptotic volume decrease?; Apoptosis,18?521
4) Liu?et al.?(2016)?Actinomycin D enhances killing of cancer cells by immunotoxin RG7787 through activation of the extrinsic pathway of apoptosis; Proc. Natl. Acad. Sci. USA,?113?10666
5) Kalousec?et al.?(2007)?Actinomycin D upregulates proapoptotic protein Puma and downregulates Bcl-2 mRNA in normal peripheral blood lymphocytes; Anticancer Drugs,?18?763
6) Matsuzaka?et al. (2016)?Characterization and functional analysis of extracellular vesicles and muscle-abundant miRNA in C2C12 myocytes and Mdx mice; PLoS One11(12)?e0167811 [Focus Biomolecules Citation] |
|