| Company Name: |
TargetMol Chemicals Inc.
|
| Tel: |
4008200310 |
| Email: |
marketing@tsbiochem.com |
| Products Intro: |
Product Name:Alimadol;Alimadol
CAS:52742-40-2
Purity:98% Package:5 mg
|
|
| | Alimadol Basic information |
| Product Name: | Alimadol | | Synonyms: | Alimadol;3-Methoxy-3,3-diphenyl-N-(2-propenyl)-1-propanamine;1,1-diphenyl-1-methoxy-3-allylaminopropane;3-methoxy-3,3-diphenyl-N-prop-2-enylpropan-1-amine;Benzenepropanamine, γ-methoxy-γ-phenyl-N-2-propen-1-yl-;A 4020;A4020;A-4020 | | CAS: | 52742-40-2 | | MF: | C19H23NO | | MW: | 281.39 | | EINECS: | | | Product Categories: | | | Mol File: | 52742-40-2.mol | 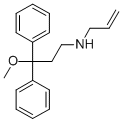 |
| | Alimadol Chemical Properties |
| Boiling point | 404.8±45.0 °C(Predicted) | | density | 1.008±0.06 g/cm3(Predicted) | | pka | 9.53±0.19(Predicted) |
| | Alimadol Usage And Synthesis |
| Originator | Alimadol ,ZYF Pharm Chemical | | Manufacturing Process | 12 g 3,3-diphenyl-3-methoxypropylamine, 13.5 g trifluoroacetic acid
anhydride, 6.0 g pyridine and 100 ml benzene was heated by stirring for 1
hour at 40°C. Then in was cooled to 0°C and poured in water. The organic
layer was separated, washed with diluted HCl and water, and evaporated to
dryness. The residue N-(3,3-diphenyl-3-methoxypropyl)trifluoroacetamide
melted at 108°-110°C. Yield: 98%.
8 g above amide was in 80 ml hexamethyl phosphoric acid amide dissolved
mixed with 1.7 g sodium hydride and stirred for 3 hours. Then 5.7 g allyl
bromide was added dropwise and the mixture was stirred for 30 minutes. It
was diluted with water and extracted with benzene. Benzene layer was
separated, evaporated to dryness. The residue was mixed with 100 ml of 75%
ethanol and 1 g sodium hydroxide and heated for 1 hour. Ethanol was distilled
off and reaction product was extracted with ether. Yield of N-(3-methoxy-3,3-
diphenylpropyl)allylamin (or 1,1-diphenyl-1-methoxy-3-allylaminopropane)
was 6.5 g (97%). MP: 40°-50°C. Hydrochloride melted at 137°-138°C.
| | Therapeutic Function | Analgesic |
| | Alimadol Preparation Products And Raw materials |
|