|
|
| | Dimoxyline Basic information |
| Product Name: | Dimoxyline | | Synonyms: | 1-[(4-Ethoxy-3-methoxyphenyl)methyl]-6,7-dimethoxy-3-methylisoquinoline;6,7-Dimethoxy-1-(4-ethoxy-3-methoxybenzyl)-3-methylisoquinoline;Paveril;Dimoxyline | | CAS: | 147-27-3 | | MF: | C22H25NO4 | | MW: | 367.443 | | EINECS: | | | Product Categories: | | | Mol File: | 147-27-3.mol | 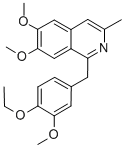 |
| | Dimoxyline Chemical Properties |
| | Dimoxyline Usage And Synthesis |
| Originator | Paveril,Lilly,US,1951 | | Definition | ChEBI: Dioxyline is a member of isoquinolines. | | Manufacturing Process | A mixture of 150 grams of 1-(3',4'-dimethoxyphenyl)-2-propanone and 70
grams of hydroxylamine hydrochloride in 125 cc of water is stirred while a
solution of 51.3 grams of sodium carbonate in 150 cc of water is added over
the course of 15 minutes, and while maintaining the reaction mixture at 30°-
40°C. The reaction mixture is stirred for an additional two and one-half hour
period at room temperature, and is then diluted with an equal volume of
water and extracted three times with 300 cc portions of ether. The combined
ether extracts are washed with water, dried over anhydrous magnesium
sulfate, and the ether is distilled off. The residue, comprising 1-(3',4'-
dimethoxyphenyl)-2-propanone oxime, may be purified by fractional
distillation in vacuo.
1-(3',4'-Dimethoxyphenyl)-2-propanone oxime thus prepared boiled at about
165°-175°C at 0.6 mm pressure. Analysis showed the presence of 7.23% of
nitrogen, compared with the calculated amount of 6.69%.
A solution of 151 grams of 1-(3',4'-dimethoxyphenyl)-2-propanone oxime in
200 cc of absolute ethanol is treated with 5 grams of Raney nickel catalyst
and ammonia in an autoclave at about 25 atm of pressure and at 75°-100°C.
The reduction is complete in about one-half hour and the reaction mixture is
filtered and fractionated under reduced pressure to recover the α-
methylhomoveratrylamine formed by the reduction. α-
Methylhomoveratrylamine thus prepared boiled at 163°-165°C at 18 mm
pressure.
A mixture of 39.0 grams (0.2mol) of α-methylhomoveratrylamine and 42.0
grams (0.2mol) of 3-methoxy-4-ethoxyphenylacetic acid is heated at 190°-
200°C for one hour. The reaction mixture is poured into about 100cc of
petroleum ether, whereupon crystals of N-(α-methylhomoveratryl)-3-methoxy-
4-ethoxyphenylacetamide separate. The precipitate is filtered off, and
recrystallized from 50% methanol-water.
N-(α-methylhomoveratryl)-3-methoxy-4-ethoxyphenylacetamide thus
prepared melted at about 135°-136°C. Analysis showed the presence of
68.05% carbon and 7.62% of hydrogen compared with the calculated amount
of 68.19% carbon and 7.54% hydrogen.
A solution of 50 grams of N-(α-methylhomoveratryl)-3-methoxy-4-
ethoxyphenylacetamide, prepared as set out above, in 200 cc of benzene, is
treated with 8 cc of phosphorus oxychloride. The mixture is refluxed for about
3 hours, cooled and then is shaken with a solution composed of 15 grams of
sodium hydroxide dissolved in 60cc of water. The aqueous layer is removed,
and the benzene solution is washed with water. The washed benzene solution
is dried over anhydrous magnesium sulfate, filtered and evaporated to
dryness. The low-melting solid residue is 6,7-dimethoxy-3-methyl-1-(3'-
methoxy-4'-ethoxybenzyl)-dihydroisoquinoline base.
To a solution of 50 grams of 6,7-dimethoxy-3-methyl-1-(4'-ethoxy-3'-
methoxybenzyl)-dihydroisoquinoline base in 200 ml of dry benzene are added
150 ml of decalin, and the mixture is distilled until its temperature reaches
180°C. 1.5 grams of 5% palladium on carbon are then added. The mixture is
stirred under reflux for about 6 hours to dehydrogenate the
dihydroisoquinoline. On cooling, the reaction mixture is diluted with petroleum
ether and the precipitated 6,7-dimethoxy-3-methyl-1-(3'-methoxy-4'-
ethoxybenzyl)-isoquinoline is filtered off and recrystallized from dilute ethanol.
6,7-Dimethoxy-3-methyl-1-(3'-methoxy-4'-ethoxybenzyl)-isoquinoline thus
prepared melted at 124°-125°C. Analysis showed the presence of 71.68%
carbon and 7.07% hydrogen as compared with the calculated amount of
71.91% carbon and 6.85% hydrogen.
A solution of 5 grams of 6,7-dimethoxy-3-methyl-1-(4'-ethoxy-3'-
methoxybenzyl)-isoquinoline in 100 cc of ethanol is treated with a solution of
1.5 grams of phosphoric acid in 10 cc of ethanol. 10 cc of water are added to
effect complete solution, and the reaction mixture is then cooled and ether is
added until precipitation of the salt is complete. The precipitate of 6,7-
dimethoxy-3-methyl-1-(3'-methoxy-4'-ethoxybenzyl)-isoquinoline phosphate is
filtered off and recrystallized from 85% ethanol by the addition of 2 volumes
of ether. | | Therapeutic Function | Vasodilator |
| | Dimoxyline Preparation Products And Raw materials |
|