|
ChemicalBook Optimization Suppliers |
|
| 融点 | 770 °C (lit.) | | 沸点 | 1420°C | | 比重(密度) | 1.98 g/mL at 25 °C (lit.) | | 屈折率 | n20/D 1.334 | | 闪点 | 1500°C | | 貯蔵温度 | 2-8°C | | 溶解性 | H2O: soluble | | 外見 | random crystals | | 比重 | 1.984 | | 色 | White | | PH | 5.5-8.0 (20℃, 50mg/mL in H2O) | | 臭い (Odor) | Odorless | | 酸塩基指示薬変色域(pH) | 7 | | 水溶解度 | 340 g/L (20 ºC) | | Sensitive | Hygroscopic | | 極大吸収波長 (λmax) | λ: 260 nm Amax: 0.02
λ: 280 nm Amax: 0.01 | | Merck | 14,7621 | | Sublimation | 1500 ºC | | BRN | 1711999 | | Dielectric constant | 4.6(Ambient) | | BCS Class | 1 | | 安定性: | Stable. Incompatible with strong oxidizing agents, strong acids. Protect from moisture. Hygroscopic. | | InChIKey | WCUXLLCKKVVCTQ-UHFFFAOYSA-M | | CAS データベース | 7447-40-7(CAS DataBase Reference) | | NISTの化学物質情報 | Potassium chloride(7447-40-7) | | EPAの化学物質情報 | Potassium chloride (7447-40-7) |
| | 塩化カリウム Usage And Synthesis |
| 外観 | 白色の結晶 | | 定義 | 本品は、カリウムの塩化物であり、次の化学式で表される。 | | 性質 | KCl(74.55).天然にはシルビン,シルビナイト,カーナル石などとして岩塩とともに産出する.これらのカリ鉱石を熱水に溶解し,その飽和水溶液を冷却し,析出させて得られる.海水中には平均0.08% 含まれる.塩化ナトリウムと同様の方法で精製する.結晶は無色の等軸晶系,塩化カリウムは,正六面体結晶.岩塩型構造.格子定数a = 0.6278 nm.K-Cl0.314 nm.苦辛味がある.融点770 ℃,沸点1505 ℃.密度1.98 g cm-3.水100 g に対する溶解度は27.6 g(0 ℃),56.7 g(100 ℃).エタノールに難溶,アセトンに不溶.カリ肥料,カリウム塩の原料として重要である.消炎火薬の消炎剤,写真用試薬,緩衝液,電極液,医薬品にも用いられる.単結晶は赤外吸収測定用のプリズムやセルの窓に用いられる. | | 解説 | カリウムと塩素の化合物。工業的には塩化カリともよばれる。天然には各種の鉱物、たとえば、シルビンKCl、シルビナイトKCl・NaCl、カーナリットKCl・MgCl2・6H2O、ハルトザルツKCl・NaCl・MgSO4・H2Oとして塩化ナトリウムや硫酸カルシウムの層間に産出する。海水中にも平均0.08%含まれている。水溶性鉱物から塩化カリウムを取り出すには連続溶解法か浮遊選鉱法が用いられる。 一例としてシルビナイトから粗塩化カリウム(含有率60~70%)を製造する工程を図に示す。再結晶法によって精製すれば高純度のものが得られる。無色の結晶性物質であるが、天然産には、不純物のために青色や黄赤色を呈するものがある。純粋なものは潮解性はないが、アルカリ土類塩などを含む粗製塩は吸湿性である。苦い辛味があり、水にはかなりよく溶けるが、アルコールやアセトンには溶けない。粗製品はそのまま肥料として用いられる。精製品は各種のカリウム塩の製造原料として重要であり、実験室では緩衝液や電極液の調製に用いられる。単結晶には、赤外線吸収スペクトル測定用のプリズムやセルの窓としての特殊な用途がある。そのほか熱処理剤、写真試薬、医薬にも使われる。 | | 溶解性 | 水に溶けやすく、エタノールに溶けにくい。 | | 解説 | 化学式はKCl。比重1.988,融点776℃,沸点1500℃。苦味と塩味がある無色の結晶。塩化カリとも。化学的性質は塩化ナトリウムに似る。天然にはシルビン(カリ岩塩)として岩塩とともに産出し,海水中にも少量存在する。工業的にはカーナライトMgCl2・KCl・6H2Oの水溶液から分別結晶により得る。カリ肥料,カリウム塩の原料,医薬などに多く用いられ,単結晶は赤外線用プリズムなどにも使われる。塩化カリは硫酸カリとともに代表的カリ肥料で,速効性である。→関連項目カリ肥料
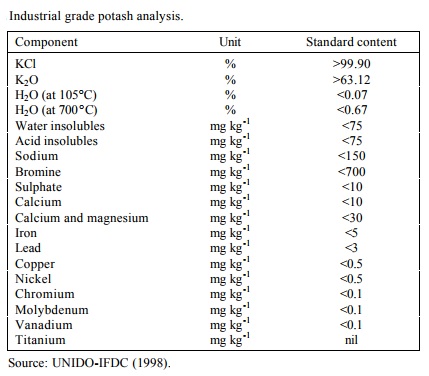
株式会社平凡社 百科事典マイペディアについて 情報 | | 用途 | カリ塩薬品原料、緩衝液、電極液、写真薬 | | 用途 | 汎用試薬。 | | 医薬用 | カリウム欠乏症の治療に医薬用の塩化カリウムが使われる。カリウムは生体内でもっとも大量に存在するイオンで、大部分が細胞内に含まれており、これが不足すると細胞の機能障害をおこす。このカリウムイオンの補給に塩化カリウムが内服または注射によって投与される。内服では胃障害を避けるために腸溶皮膜を施した徐放性製剤が用いられる。「スローケー」などがそれである。注射では電解質補正用や心臓手術の際の心停止液として輸液に混ぜて使われる。 | | 用途 | 汎用試薬、調製液原料、製剤原料。 | | 用途 | JIS K0130 電気伝導率測定方法通則中の塩化カリウム標準液の調製。 | | 用途 | 高純度金属化合物。 | | 化粧品の成分用途 | 親水性増粘剤 | | 効能 | カリウム補充薬, 補正用電解質液 | | 商品名 | K.C.L. (丸石製薬); K.C.L. (丸石製薬); KCL (大塚製薬工場); KCL (大塚製薬工場); スローケー (ノバルティスファーマ); 塩化カリウム (山善製薬); 塩化カリウム (扶桑薬品工業); 塩化カリウム (日医工ファーマ) | | 使用上の注意 | 純度は金属ベースで差数法によって算出したもので、重量又は容量分析等の化学的方法によるものではありません。使用目的により、正確な含量が必要な場合は、それらの方法によって測定する必要があります。 | | 説明 | Potassium chloride (KCl) is a metal halide salt that is used in a variety of areas. The dominant application of potassium chloride is to serve as a fertilizer, which offers potassium to plants and prevents them from certain diseases. Besides, it can be applied in food and medical industry. As a treatment for hypokalemia, potassium chloride pills are taken to balance the blood's potassium levels and prevent potassium deficiency in the blood. In food industry, it serves as a electrolyte replenisher and a good salt substitute for food, as well as a firming agent to give consistent texture to food, thus to strengthen its structure. | | 化学的特性 | Potassium chloride occurs as odorless, colorless crystals or a white crystalline powder, with an unpleasant, saline taste. The crystal lattice is a face-centered cubic structure. Potassium chloride occurs naturally as the mineralsylvite (KCl) and as carnallite(KCl·MgCl2·6H2O); it is produced industriallyby fractional crystallizationof these deposits or of solutions fromlake brines. It has the interesting property of being more soluble than sodium chloride in hot water but less soluble in cold. It has low toxicity. | | 使用 | About 4-5% of potash production is used in industrial applications (UNIDOIFDC, 1998). In 1996, the world supply of industrial grade potash was close to 1.35 Mt K2O. This industrial material is 98-99% pure, compared with the agricultural potash specification of 60% K2O minimum (equivalent to 95% KCl). Industrial potash should contain at least 62% K2O and have very low levels of Na, Mg, Ca, SO4 and Br. This high-grade potash is produced by only a few producers in worldwide.
Potassium hydroxide (KOH), also known as caustic potash, is the largestvolume K product for non-fertilizer use. It is produced by the electrolysis of industrial KCl and is widely used for manufacturing soaps, detergents, grease, catalysts, synthetic rubber, matches, dyes and insecticides. Caustic potash is also as a liquid fertilizer and as an ingredient in alkaline batteries and photographic film processing chemicals.
Potassium hydroxide is a raw material in the production of various K salts, mainly K carbonates, and also citrates, silicates, acetates, etc. Potassium carbonate confers excellent clarity to glass thus is used for most fine optical lenses, eyeglasses, fine crystal, glassware, chinaware and TV tubes. Potassium bicarbonate is used largely in the food and pharmaceutical industries.
Potash-derived compounds and salts are also used in the production of metal fluxes, cured meats, tempered steel, paper fumigants, case hardened steel, bleaching agents, baking powder, cream of tartar and beverages. Worldwide, industrial KCl is estimated to be used as follows: detergents and soaps, 30-35%; glass and ceramics, 25-28%; textiles and dyes 20-22%; chemicals and drugs, 13-15%; and other uses, 7-5% (UNIDO-IFDC, 1998). | | 使用 | Potassium chloride (KCl), commonly referred to as muriate of potash, is the most common source of potash (K2O), and accounts for about 95 % of world potash production. Virtually all (90 %) commercial potash is extracted from natural sources of potassium salt deposits occurring in thin beds in large salt basins formed by the evaporation of ancient seas. Present-day salt lakes and natural brines represent about 10 % of total recoverable potash. Extraction is followed by milling, washing, screening, flotation, crystallization, refining and drying.
More than 90 % of the total KCl consumption is used for fertilizer production. Production of potassium hydroxide accounts for more than 90 % of the non-fertilizer or industrial use of KCl. KOH is also used in the production of some agricultural-grade liquid fertilizers. uses of KCl include:
- Potassium chloride (KCl) is inorganic salt used for making fertilizers, since the growth of many plants is limited by their potassium intake. Potassium in plants is important for the osmotic and ionic regulation, plays a key role in the water homeostasis and is closely connected with processes involved in the protein synthesis.
- In photography. In buffer solutions, electrode cells.
- Potassium chloride may be used for the preparation of phosphate buffered saline, and for the extraction and solubilization of proteins.
- Used in buffer solutions, medicine, scientific applications, and food processing.
- Used in nutritent; gelling agent; salt substitute; yeast food.
- food/foodstuff additives: KCl is used as a nutrient and/or dietary supplement food additive. KCl also serves as a potassium supplement of animal feed.
- pharmaceutical products: KCl is an important therapeutic agent, which is used mainly in the treatment of hypokalemia and associated conditions. Hypokalemia (potassium deficiency) is a potentially fatal condition in which the body fails to retain sufficient potassium to maintain health.
- laboratory chemicals: KCl is used in electrode cells, buffer solutions, and spectroscopy.
- drilling mud for oil production industry: KCl is used as conditioner in oil drilling muds and as a shale stabilizer to prevent swelling.
- flame retardants and fire preventing agents: KCl is used as a component in dry chemical fire extinguisher.
- anti-freezing agents: KCl is used to melt ice on streets and driveways.
| | 使用 |
- Potassium chloride is a widely used reagent in biochemistry and molecular biology. It is a component of phosphate buffered saline (PBS, Product No. P 3813) and of polymerase chain reaction (PCR) buffer (50 mM KCl).
- KCl is also used in studies of ion transport and potassium channels.
- KCl is also utilized in the solubilization, extraction, purification, and crystallization of proteins.
- The use of KCl in the crystallization of histone core octamers has been reported.
| | 使用 | Potassium Chloride is a nutrient, dietary supplement, and gelling
agent that exists as crystals or powder. it has a solubility of 1 g in
2.8 ml of water at 25°c and 1 g in 1.8 ml of boiling water. hydrochloric
acid, and sodium chloride and magnesium chloride diminish its
solubility in water. it is used as a salt substitute and mineral supple-
ment. it has optional use in artificially sweetened jelly and preserves.
it is used as a potassium source for certain types of carrageenan gels.
it is used to replace sodium chloride in low-sodium foods. | | 調製方法 | Potassium chloride occurs naturally as the mineral sylvite or sylvine;
it also occurs in other minerals such as sylvinite, carnallite, and
kainite. Commercially, potassium chloride is obtained by the solar
evaporation of brine or by the mining of mineral deposits. | | 定義 | ChEBI: Potassium chloride is a metal chloride salt with a K(+) counterion. It has a role as a fertilizer. It is a potassium salt, an inorganic chloride and an inorganic potassium salt. | | brand name | Apo-k;Celeka;Durules-k;Kadalex;Kalinorm;Kalipor;Kalium durules;K-long;Miopotasio;Plenish-k;Potasion;Roychlor;Rum-k;Swiss-kal sr;Ultra-k-chlor. | | 世界保健機関(WHO) | Potassium chloride has been used for many years to correct
potassium deficiency. The use of fast-acting tablets has been associated with
lesions of the gastro-intestinal mucosa, which have led to their general withdrawal. | | 一般的な説明 | Potassium chloride (KCl) is a water-soluble metal salt that comprises of potassium and chlorine. It can be extracted from minerals and salt water. KCl can be used in industries such as cosmetics, food, biomedical, chemical and fertilizer. | | 空気と水の反応 | Hygroscopic. Water soluble. | | 反応プロフィール | Potassium chloride is not in general strongly reactive. Violent reaction with BrF3 and with a mixture of sulfuric acid potassium permanganate mixture . Reacts with concentrated sulfuric acid to generate fumes of hydrogen chloride. | | 健康ハザード | Potassium chloride is an essential constituent of the body for intracellular osmotic pressure and buffering, cell permeability, acid-base balance, muscle contraction and nerve function.
SYMPTOMS: Large doses of Potassium chloride usually induce vomiting, so acute intoxication by mouth is rare. If no pre-existing kidney damage, it is rapidly excreted. Poisoning disturbs the rhythm of heart. Large doses by mouth can cause gastrointestinal irritation, purging, weakness, and circulatory disturbances. | | 火災危険 | Flammability data is not available, but Potassium chloride is probably nonflammable. | | 燃焼性と爆発性 | Non flammable | | 农业用途 | Muriate of potash or potassium chloride (KCl), is a major
potash fertilizer. It is water soluble and is generally
blended with other components to make it a multi-nutrient
fertilizer. It has a higher salt index than
potassium sulphate and is recommended for most crops
except tobacco, potato and grapes, which are sensitive to
chloride ions. | | 応用例(製薬) | Potassium chloride is widely used in a variety of parenteral and
nonparenteral pharmaceutical formulations. Its primary use, in
parenteral and ophthalmic preparations, is to produce isotonic
solutions.
Potassium chloride is also used therapeutically in the treatment
of hypokalemia.
Many solid-dosage forms of potassium chloride exist including:
tablets prepared by direct compression and granulation;
effervescent tablets; coated, sustained-release tablets; sustained-
release wax matrix tablets;microcapsules;pellets;
and osmotic pump formulations.
Experimentally, potassium chloride is frequently used as a model
drug in the development of new solid-dosage forms, particularly for
sustained-release or modified-release products.
Potassium chloride is also used widely in the food industry as a
dietary supplement, pH control agent, stabilizer, thickener, and
gelling agent. It can also be used in infant formulations. | | 农业用途 | Potassium chloride (KCl), also known as muriate of
potash, is generally blended with other components to
make it a multinutrient fertilizer. It is a white crystalline
solid, available in fine, coarse and granular grades. It is
the least expensive carrier of potassium in the fertilizer
market. This important fertilizer contains about 48 to
52% plant food as potassium and about 48% chloride.
Coarser potassium blends well with granular N-P
compounds to form an NPK-blended multinutrient
fertilizer.
At least 78 % of the potassium salts are estimated to be
consumed worldwide, in the form of potassium chloride,
and over 90% of all processed potassium is used as
fertilizer. Muck, peat and sands are generally potassiumdeficient,
whereas arid soils are mostly potassium-rich,
with 448 kg/ha or more of readily available potassium.
Potassium chloride is neutral and totally watersoluble.
It can be applied to all soils and crops that are not
sensitive to chlorides. Soluble soil-potassium is adsorbed
and retained by soil colloids and thus prevented from
leaching. Roots take up potassium in the ionic form.
Potassium chloride is best applied either while sowing
or prior to it. However, when soils are light or coarsetextured,
the applied potassium may be lost through
leaching. So, it is preferable to apply potassium in split
doses. On heavy soils, the fertilizer is placed advantageously in bands, as in the case of phosphatic fertilizers.
Potassium chloride is manufactured from potash
minerals or brine. Sylvinite, which is a mixture of
potassium chloride and halite, is the major potash
mineral used for potassium chloride manufacture. A
large percentage of potassium chloride is mined and
refined either by the floatation or crystallization process.
Both processes, of which the floatation process is more
common, involve the separation of potassium chloride
from sodium chloride. Fine potassium chloride is a freeflowing
material which does not cake in dry places. | | 工業用途 | Potassium chloride is a colorless or white crystallinecompound of the composition KCl, usedfor molten salt baths for the heat treatment ofsteels. The specific gravity is 1.987. A bathcomposed of three parts potassium chloride andtwo parts barium chloride is used for hardeningcarbon-steel drills and other tools. Steel toolsheated in this bath and quenched in a 3% sulfuricacid solution have a very bright surface.A common bath is made up of potassium chlorideand common salt and can be used for temperaturesup to 900°C.
Potassium chloride is used in the porcelainenamel industry as a setting-up agent in titaniumcover coats. In general, the quantities ofpotassium chloride, when used as an electrolyte,will be approximately the same as sodiumnitrite, which it replaces. However, KCl doesnot aid tearing resistance as does nitrite. Themain advantage in using potassium chloride isthe freedom from yellowing or creaming whenused in a blue-white enamel. Potassium chloridemay exert an adverse effect on the glossand may cause a slight decrease in the acidresistingproperties of the enamel, although thelatter effect is somewhat debatable. | | 臨床応用 | Hypokalaemia | | 安全性プロファイル | A human poison by
ingestion. Poison experimentally by
ingestion, intravenous, and intraperitoneal
routes. Human systemic effects by ingestion:
nausea, blood clotting changes, carhac
arrhythmias. An eye irritant. Mutation data
reported. Explosive reaction with BrF3;
sulfuric acid + potassium permanganate.
When heated to decomposition it emits
toxic fumes of K2O and Cl-. | | 安全性 | Potassium chloride is used in a large number of pharmaceutical
formulations, including oral, parenteral, and topical preparations,
both as an excipient and as a therapeutic agent.
Potassium ions play an important role in cellular metabolism
and imbalances can result in serious clinical effects. Orally ingested
potassium chloride is rapidly absorbed from the gastrointestinal
tract and excreted by the kidneys. Potassium chloride is more
irritant than sodium chloride when adminstered orally, and
ingestion of large quantities of potassium chloride can cause effects
such as gastrointestinal irritation, nausea, vomiting, and diarrhea.
High localized concentrations of potassium chloride in the
gastrointestinal tract can cause ulceration: hence the development of
the many enteric-coated and wax matrix sustained-release preparations
that are available.Although it is claimed that some
formulations cause less ulceration than others, it is often preferred
to administer potassium chloride as an aqueous solution. However,
solutions have also been associated with problems, mainly due to
their unpleasant taste.
Parenterally, rapid injection of strong potassium chloride
solutions can cause cardiac arrest; in the adult, solutions should
be infused at a rate not greater than 750 mg/hour.
Therapeutically, in adults, up to 10 g orally, in divided doses has
been administered daily, while intravenously up to 6 g daily has
been used.
(guinea pig, oral): 2.5 g/kg
(mouse, IP): 1.18 g/kg
(mouse, IV): 0.12 g/kg
(mouse, oral): 0.38 g/kg
(rat, IP): 0.66 g/kg
(rat, IV): 0.14 g/kg
(rat, oral): 2.6 g/kg | | 薬物相互作用 | Potentially hazardous interactions with other drugs
ACE inhibitors and angiotensin-II antagonists:
increased risk of hyperkalaemia.
Ciclosporin: increased risk of hyperkalaemia.
Potassium-sparing diuretics: increased risk of
hyperkalaemia.
Tacrolimus: increased risk of hyperkalaemia. | | 代謝 | Potassium is excreted mainly by the kidneys; it is secreted
in the distal tubules in exchange for sodium or hydrogen
ions. Some potassium is excreted in the faeces and small
amounts may also be excreted in sweat. | | 貯蔵 | Potassium chloride tablets become increasingly hard on storage at
low humidities. However, tablets stored at 76% relative humidity
showed no increase or only a slight increase in hardness.The
addition of lubricants, such as 2% w/w magnesium stearate,
reduces tablet hardness and hardness on aging.Aqueous
potassium chloride solutions may be sterilized by autoclaving or
by filtration.
Potassium chloride is stable and should be stored in a well-closed
container in a cool, dry place. | | 純化方法 | Dissolve it in conductivity water, filter it, and saturate it with chlorine (generated from conc HCl and KMnO4). Excess chlorine is boiled off, and the KCl is precipitated by HCl (generated by dropping conc HCl into conc H2SO4). The precipitate is washed with water, dissolved in conductivity water at 90-95o, and crystallised by cooling to about -5o. The crystals are drained at the centrifuge, dried in a vacuum desiccator at room temperature, then fused in a platinum dish under N2, cooled and stored in a desiccator. Potassium chloride has also been sublimed in a stream of pre-purified N2 gas and collected by electrostatic discharge [Craig & McIntosh Can J Chem 30 448 1952]. | | 不和合性 | Potassium chloride reacts violently with bromine trifluoride and
with a mixture of sulfuric acid and potassium permanganate. The
presence of hydrochloric acid, sodium chloride, and magnesium
chloride decreases the solubility of potassium chloride in water.
Aqueous solutions of potassium chloride form precipitates with
lead and silver salts.
Intravenous aqueous potassium chloride solutions are incompatible
with protein hydrolysate. | | 規制状況(Regulatory Status) | GRAS listed. Accepted as a food additive in Europe. Included in the
FDA Inactive Ingredients Database (injections, ophthalmic preparations,
oral capsules, and tablets). Included in nonparenteral and
parenteral medicines licensed in the UK. Included in the Canadian
List of Acceptable Non-medicinal Ingredients. | | 参考文献 | https://en.wikipedia.org/wiki/Potassium_chloride
https://www.drugbank.ca/drugs/DB00761
http://study.com/academy/lesson/what-is-potassium-chloride-uses-formula-side-effects.html |
|