|
ChemicalBook Optimization Suppliers |
| 名前: |
LGM Pharma |
| 電話番号: |
1-(800)-881-8210 |
| 電子メール: |
inquiries@lgmpharma.com |
|
| 化学名: | アンブロキソール塩酸塩 | | 英語化学名: | Ambroxol | | 别名: | 4-((2-amino-3,5-dibromobenzyl)amino)-(e)-cyclohexano;5-dibromophenyl)methyl)amino)-4-(((2-amino-trans-cyclohexano;Cyclohexanol, 4-((2-amino-3,5-dibromobenzyl)amino)- (E)-;Cyclohexanol, 4-[[(2-amino-3,5-dibromophenyl)methyl]amino]-, trans-;n-(2-amino-3,4-dibromociclohexil)-trans-4-aminociclohexanol;n-(2-amino-3,4-dibromocyclohexyl)-trans-4-aminocyclohexanol;N-(trans-4-Hidroxiciclohexil)-(2-amino-3,5-dibromobencil)amina;N-(trans-4-Hydroxycyclohexyl)-(2-amino-3,5-dibromobenzyl)-amine | | CAS番号: | 18683-91-5 | | 分子式: | C13H18Br2N2O | | 分子量: | 378.1 | | EINECS: | 242-500-3 | | カテゴリ情報: | API | | Mol File: | 18683-91-5.mol | 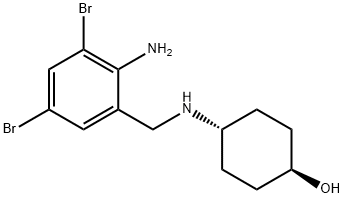 |
| 融点 | 233-234 C | | 沸点 | 468.6±45.0 °C(Predicted) | | 比重(密度) | 1.5863 (rough estimate) | | 屈折率 | 1.6220 (estimate) | | 貯蔵温度 | Keep in dark place,Sealed in dry,2-8°C | | 溶解性 | Soluble in Water. | | 外見 | solid | | 酸解離定数(Pka) | 15.12±0.40(Predicted) | | 色 | White | | 安定性: | Stable for 1 year from date of purchase as supplied. Solutions in distilled water may be stored at -20° for up to 3 months. | | CAS データベース | 18683-91-5(CAS DataBase Reference) | | NISTの化学物質情報 | Ambroxol(18683-91-5) |
| 主な危険性 | Xn,Xi | | Rフレーズ | 22-36/37/38 | | Sフレーズ | 36-26 | | WGK Germany | 3 | | RTECS 番号 | GV8423000 | | 毒性 | guinea pig,LD50,intraperitoneal,280mg/kg (280mg/kg),Medicamentos de Actualidad. Vol. 15, Pg. 523, 1979. |
| | アンブロキソール塩酸塩 Usage And Synthesis |
| 用途 | アンブロキソール(英語?ドイツ語: Ambroxol)は、去痰薬に属する医薬品の1種であり、痰の喀出に困難を伴う急性および慢性の下部気道症状の治療、および急性の咽頭部痛を緩和するのに用いられる。 | | 用途 | 肺胞表面活性代謝物の分泌を 増加します。 | | 効能 | 去痰薬, 粘液溶解薬 | | 説明 | Ambroxol HCl (18683-91-5) is a radical scavenger with anti-inflammatory activity. Blocks the generation of reactive oxygen species by bronchoalveolar lavage cells, inhibits doxorubicin-induced lipid peroxidation (70 mg/kg in rat), and inhibits the release of inflammatory mediators from human leukocytes and mast cells (effective dose ~ 100 μM) | | Originator | Mucosolvan,Thomae,W. Germany,1980 | | 使用 | Ambroxol is a metabolite of Bromohexine. Ambroxol Hydrochloride is a bronchosecretolytic drug. Free base of Ambroxol Hydrochloride (A575900). | | 使用 | expectorant | | 定義 | ChEBI: Ambroxol is an aromatic amine. | | Manufacturing Process | 6.5 g of N-(trans-p-hydroxycyclohexyl)-(2-aminobenzyl)-amine were dissolved
in a mixture of 80 cc of glacial acetic acid and 20 cc of water, and then 9.6 g
of bromine were added dropwise at room temperature while stirring the
solution. After all of the bromine had been added, the reaction mixture was
stirred for two hours more and was then concentrated in a water aspirator
vacuum. The residue was taken up in 2 N ammonia, the solution was
extracted several times with chloroform, and the organic extract solutions
were combined and evaporated. The residue, raw N-(trans-phydroxycyclohexyl)-(
2-amino-3,5-dibromobenzyl)-amine, was purified with
chloroform and ethyl acetate over silica gel in a chromatographic column, the
purified product was dissolved in a mixture of ethanol and ether, and the
solution was acidified with concentrated hydrochloric acid. The precipitate
formed thereby was collected and recrystallized from ethanol and ether,
yielding N-(trans-p-hydroxycyclohexyl)-(2-amino-3,5-dibromobenzyl)-amine
hydrochloride, MP 233-234.5°C (decomposition). | | Therapeutic Function | Expectorant | | 参考文献 | 1) Teramoto et al. (1999), Effects of ambroxol on spontaneous or stimulated generation of reactive oxygen species by bronchoalveolar lavage cells harvested from patients with or without chronic obstructive pulmonary diseases; Pharmacology, 59 135
2) Nowak et al. (1995), Ambroxol inhibits doxorubicin-induced lipid peroxidation in heart of mice; Free Radic. Biol. Med., 19 659
3) Gibbs et al. (1999), Ambroxol inhibits the release of histamine, leukotrienes and cytokines from human leukocytes and mast cells; Inflamm. Res., 48 86 |
|