|
ChemicalBook Optimization Suppliers |
|
| | cis-ジアンミン白金(II)ジクロリド 製品概要 |
| | cis-ジアンミン白金(II)ジクロリド 物理性質 |
| 融点 | 270 °C (lit.) | | 比重(密度) | 3,7 g/cm3 | | 貯蔵温度 | 2-8°C | | 溶解性 | Soluble in DMF. Insoluble in most common solvents | | 外見 | crystalline | | 色 | yellow | | 水溶解度 | <0.1 g/100 mL at 19 ºC | | Merck | 14,2317 | | 暴露限界値 | ACGIH: TWA 0.002 mg/m3
NIOSH: IDLH 4 mg/m3; TWA 0.002 mg/m3 | | 安定性: | Stable. Incompatible with oxidizing agents, aluminium, antioxidants. | | CAS データベース | 15663-27-1(CAS DataBase Reference) | | IARC | 2A (Vol. 26, Sup 7) 1987, 1 (Vol. 76, 100A) 2012 | | EPAの化学物質情報 | Cisplatin (15663-27-1) |
| | cis-ジアンミン白金(II)ジクロリド Usage And Synthesis |
| 外観 | わずかにうすい黄色〜黄色, 結晶性粉末〜粉末 | | 溶解性 | 水に溶けにくく、エタノール及びアセトンにほとんど溶けない。[溶解方法] 0.9% NaCl溶液に0.5mg/mlに溶解。使用時は実験に使用する溶液で希釈を行う。 | | 解説 | シスプラチン,睾丸腫瘍,膀胱癌,卵巣癌などの化学療法剤として中心的な薬剤であり,構造式に白金を含む薬物。しかし,腎毒性や催吐作用など副作用も強いことがネックとなっていた。シスプラチンの副作用を軽減するために,カルボプラチンが開発され,日本では 1990年3月に承認が得られた。カルボプラチンは,すでに世界 30ヵ国で使用されており,シスプラチン投与時に必要な大量の水分負荷が不要なこと,蓄積毒性が少なく継続治療が可能であることから,その治療効果が注目されている。白金錯体 [PtCl2(NH3)2]2+ のシス体.睾丸がん,卵巣がん,乳がんなどに有効であるが,トランス体には抗がん性はない.類似白金化合物として,カルボフラチンが臨床に用いられている.DNAに結合し,その複製を阻害する. | | 用途 | 様々ながんに使用される細胞増殖抑制剤、抗がん剤 | | 用途 | がん研究用試薬。 | | 用途 | DNA 鎖内及び鎖間の白金
-DNA 架橋を形成し、DNA の複製及び転写阻
害作用を示します。 | | 効能 | 抗悪性腫瘍薬, 細胞増殖阻害薬 | | 商品名 | アイエーコール (日本化薬); ランダ (日本化薬) | | 使用上の注意 | 不活性ガス封入 | | 説明 | Cisplastin is an non-organic platinum-containing drug with alkylating properties. It causes
cross-linking of DNA and RNA chains. In particular, it has been shown, that cisplastin, like
other alkylating agents, bind primarily at N7 of two neighboring deoxyguanylates to DNA,
which inhibits its replication. It is only used intravenously. It is highly reactive with carci�nomas of the testicles, ovaries, heat, neck, spleen, lungs, and so on. | | 化学的特性 | Cisplatin is a white powder or yellow crystalline solid with the melting point 268-272°C (decomposition). It is slightly soluble in water and easily soluble in dimethylformamide. In aqueous solution, it can be gradually transformed into trans-and hydrolysis. | | Originator | Blastolem,Lemery,Mexico | | 使用 | Cisplatin is a cytostatic agent and it is used to treat various
cancer types, including cancer of ovary, testis, lung, head,
neck, bladder, neuroblastoma, and nephroblastoma, and
Hodgkin’s disease and non-Hodgkin lymphoma. | | 使用 | cisplatin is a platinum complex, cis-[PtCl2(NH3)2], used in cancer treatmentto inhibit the growth oftumours. It acts by binding betweenstrands of DNA. | | 定義 | ChEBI: Cisplatin is a diamminedichloroplatinum compound in which the two ammine ligands and two chloro ligands are oriented in a cis planar configuration around the central platinum ion. An anticancer drug that interacts with, and forms cross-links between, D A and proteins, it is used as a neoplasm inhibitor to treat solid tumours, primarily of the testis and ovary. | | 調製方法 | Cisplatin is obtained by the method described by Kauffman
and Cowan, in which potassium(II) tetrachloroplatinate
is treated with buffered aqueous ammonia solution.
Pure cisplatin is obtained by recrystallization from dilute
hydrochloric acid. | | 適応症 | Cisplatin (Platinol) is an inorganic coordination complex
with a broad range of antitumor activity. It is especially
useful in the treatment of testicular and ovarian
cancer. It binds to DNA at nucleophilic sites, such as the
N7 and O6 of guanine, producing alterations in DNA
structure and inhibition of DNA synthesis. Adjacent
guanine residues on the same DNA strand are preferentially
cross-linked. This platinating activity is analogous
to the mode of action of alkylating agents. Cisplatin also
binds extensively to proteins. It does not appear to be
phase specific in the cell cycle. | | Manufacturing Process | The synthesis proceeds dy reduction of potassium hexachlorplatinate with
hydrazine to give potassium tetrachloroplatinate. This is converted to
potassium tetraiodoplatinate by treatment with potassium iodide and then
reacted with 6 M ammonium hydroxide to give crystals of cisplatin | | Therapeutic Function | Antitumor | | 一般的な説明 | administrationin the treatment of a wide variety of cancers includingnon-Hodgkin’s lymphoma, bladder cancer, ovarian cancer,testicular cancer, and cancers of the head and neck. A liposomalform is also available as well as an injectable collagenmatrix gel containing cisplatin. Compared with other platins,cisplatin is the most reactive and therefore the most effectivein platinating DNA. After IV administration, the agent iswidely distributed, highly protein bound (90%), and concentratesin the liver and kidney. After infusion, covalent attachmentto plasma proteins occurs such that after 4 hours, 90%of drug is protein bound. The elimination of platinum fromthe blood is a slow process with a terminal elimination halflifeof 5 to 10 days. Metabolism involves aquation, which occursto a greater extent once distribution out of the plasmahas occurred. Additional metabolites have been seen resultingfrom reaction with glutathione and cysteine. The greaterreactivity of cisplatin gives rise to significant toxicitiescompared with other platins. These include dose-limitingnephrotoxicity, which normally presents as elevated bloodurea nitrogen (BUN) and creatinine. This effect is cumulativeupon repeated dosing and may progress further to necrosis,altered epithelial cells, cast formation, and thickening ofthe tubular basement membranes but is generally reversibleupon discontinuation of drug treatment. Sodium thiosulfatemay be given to reduce the nephrotoxicity. Neurotoxicitymay also be dose limiting, normally presenting initially asnumbness but may progress to seizure. Other adverse effectsinclude myelosuppression, nausea, vomiting, alopecia,ototoxicity, ocular toxicity, azoospermia, impotence, myocardialinfarction, thrombotic events, and inappropriatesecretion of antidiuretic hormone. | | 一般的な説明 | An anticancer drug. Orange-yellow to deep yellow solid or powder. | | 空気と水の反応 | Insoluble in water. | | 反応プロフィール | Cisplatin is incompatible with oxidizing agents. Cisplatin is also incompatible with aluminum. Cisplatin may react with sodium bisulfite and other antioxidants. | | 火災危険 | Flash point data for Cisplatin are not available; however, Cisplatin is probably combustible. | | 応用例(製薬) | CDDP, also referred to as cisplatinum or cisplatin, is a yellow powder and has found widespread use a
chemotherapeutic agent. | | 生物活性 | Cisplatin is a platinum-containing compound that acts as a DNA-crosslinking agent and interferes with replication and transcription, culminating in apoptosis. It forms intra- and interstrand crosslinks with DNA with intrastrand guanine-to-guanine or guanine-to-alanine links accounting for the majority of DNA binding. Cisplatin halts the cell cycle at the G2/M phase in vitro and is active against murine tumors transplanted into mice and in mouse xenograft models, including a reduction in tumor growth in a model of squamous cell carcinoma of the head and neck when administered at doses ranging from 7.5 to 12.5 mg/kg. Cisplatin also inhibits the RecA recombinase of M. tuberculosis (IC50 = 2 μM), blocking protein splicing and cell growth. Formulations containing cisplatin have been used, alone and in combination therapy, in the treatment of a variety of cancers. | | Biochem/physiol Actions | Potent platinum-based antineoplastic agent. Forms cytotoxic adducts with the DNA dinucleotide d(pGpG), inducing intrastrand cross-links. | | 作用機序 | Cisplatin shows biphasic plasma decay with a distribution
phase half-life of 25 to 49 minutes and an elimination
half-life of 2 to 4 days. More than 90% of the
drug is bound to plasma proteins, and binding may approach
100% during prolonged infusion. Cisplatin does
not cross the blood-brain barrier. Excretion is predominantly
renal and is incomplete. | | 臨床応用 | Cisplatin, combined with bleomycin and vinblastine
or etoposide, produces cures in most patients with
metastatic testicular cancer or germ cell cancer of the
ovary. Cisplatin also shows some activity against carcinomas
of the head and neck, bladder, cervix, prostate,
and lung. | | 副作用 | Renal toxicity is the major potential toxicity of
cisplatin. Severe nausea and vomiting that often accompany
cisplatin administration may necessitate hospitalization.
Cisplatin has mild bone marrow toxicity, yielding
both leukopenia and thrombocytopenia. Anemia is
common and may require transfusions of red blood
cells. Anaphylactic allergic reactions have been described.
Hearing loss in the high frequencies (4000 Hz)
may occur in 10 to 30% of patients. Other reported toxicities
include peripheral neuropathies with paresthesias,
leg weakness, and tremors. Excessive urinary excretion
of magnesium also may occur. | | 安全性プロファイル | Confirmed carcinogen
with experimental carcinogenic and
tumorigenic data. Poison by ingestion,
intramuscular, submtaneous, intravenous,
and intraperitoneal routes. Human systemic
effects: change in audttory acuity, change in
kidney tubules, changes in bone marrow,
corrosive to skin, depressed renal function
tests, hallucinations, nausea or vomiting.
Experimental teratogenic and reproductive
effects. Human mutation data reported.
When heated to decomposition it emits very
toxic fumes of Cland NOx. See also
PLATINUM COMPOUNDS. | | 合成 | Cisplatin, cis-diaminodichloroplatinum (30.2.5.1), is made by reducing potas�sium hexachloroplatinate by hydrazine to potassium tetrachloroplatinate, which reacts
with ammonia to give cisplatin (30.2.5.1) . 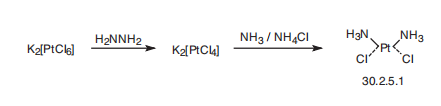
| | 職業ばく露 | A potential danger to those involved in the manufacture, formulation and administration of this anticancer chemotherapy agent. Contact with water causes decomposition. | | Veterinary Drugs and Treatments | In veterinary medicine, the systemic use of cisplatin is presently
limited to use in dogs. The drug has been, or may be, useful in a
variety of neoplastic diseases including squamous cell carcinomas,
transitional cell carcinomas, ovarian carcinomas, mediastinal carcinomas,
osteosarcomas, pleural adenocarcinomas, nasal carcinomas,
and thyroid adenocarcinomas.
Cisplatin may be useful for the palliative control of neoplastic
pulmonary effusions after intracavitary
administration.
In horses, cisplatin has been used for intralesional injection for
skin tumors. | | 薬物相互作用 | Potentially hazardous interactions with other drugs
Aldesleukin: avoid concomitant use.
Antibacterials: increased risk of nephrotoxicity
and possibly ototoxicity with aminoglycosides,
capreomycin, polymyxins or vancomycin.
Antipsychotics: avoid with clozapine, increased risk
of agranulocytosis.
Cytotoxics: increased risk of ototoxicity with
ifosfamide; increased pulmonary toxicity with
bleomycin and methotrexate. | | 応急処置 | If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit. | | 発がん性 | Cisplatin is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals. | | 代謝 | It is rapidly hydrated, resulting in a short plasma half-life of less than 30 minutes. It is eliminated predominantly via the kidney, but approximately 10% of a given dose undergoes biliary excretion. It is highly nephrotoxic and can cause significant damage to the renal tubules, especially in patients with preexisting kidney disease or one kidney or who are concurrently receiving other nephrotoxic drugs (e.g., cyclophosphamide or ifosfamide). Dosages should be reduced in any of the above situations. Clearance decreases with chronic therapy, and toxicities can manifest at a late date. To proactively protect patients against kidney damage, patients should be hydrated with chloride-containing solutions. Saline or mannitol diuretics can be administered to promote continuous excretion of the drug and its hydrated analogues. Sodium thiosulfate, which accumulates in the renal tubules, also has been used to neutralize active drug in the kidneys in an effort to avoid nephrotoxicity. | | 貯蔵 | Room temperature | | 輸送方法 | UN2928 Toxic solids, corrosive, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, 8-Corrosive material, Technical Name Required. UN3290 Toxic solid, corrosive, inorganic, n.o.s., Hazard class: 6.1; Labels: 6.1-Poisonous materials, 8-Corrosive material. UN3288 Toxic solids, inorganic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN3249 Medicine, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials | | 純化方法 | Recrystallise it from dimethylformamide and check the purity by IR and UV-VIS spectroscopy. [Raudaschl et al. Inorg Chim Acta 78 143 1983.] HIGHLY TOXIC, SUSPECTED CARCINOGEN. | | 不和合性 | Aluminum reacts with cisplatin and decreases the drug’s effectiveness. Do not use any aluminum equipment to prepare or administer cisplatin. | | 廃棄物の処理 | Disposal of unused product must be undertaken by qualified personnel who are knowledgeable in all applicable regulations and follow all pertinent safety precautions including the use of appropriate protective equipment. For proper handling and disposal, always comply with federal, state, and local regulations | | 参考文献 | 1) Van Waardenburg et al. (2004), Platinated DNA adducts enhance poisoning of DNA topoisomerase I by camptothecin; J. Biol. Chem,, 279 54502 DOI:10.1074/JBC.M410103200
2) Siddik et al. (2003), Cisplatin: mode of cytotoxic action and molecular basis of resistance; Oncogene, 22 7265 DOI:10.1038/sj.onc.1206933
3) Seki et al. (2000), Cisplatin (CDDP) specifically induces apoptosis via sequential activation of caspase-8, -3 and -6 in osteosarcoma; Cancer Chemother. Pharmacol., 45 199 DOI:10.1007/s002800050030
4) Nomura et al. (2004), Cisplatin inhibits the expression of X-linked inhibitor of apoptosis protein in human LNCaP cells; Urol. Oncol., 22 453 DOI:10.1016/J.UROLONC.2004.04.035
5) Raghavan et al. (2015), Dimethylsulfoxide inactivates the anticancer effect of cisplatin against myelogenous leukemia cell lines in in vitro assays.; Indian J. Phamracol., 47 322 DOI:10.4103/0253-7613.157132
6) Synthesis of Essential Drugs (2006, Elsevier) - libgen.lc
7) Sittig's Pharmaceutical Manufacturing Encyclopedia
8) Patty's Toxicology 6-Volume Set-Wiley (2012)
9) Modern pharmacology with clinical applications (2004, LWW) |
|