| Company Name: |
JW & Y Pharmlab Co., Ltd.
|
| Tel: |
021-64340559 13651849907; |
| Email: |
xinyu_shi@jwypharmlab.com.cn |
| Products Intro: |
Product Name:5-Hydroxymethyl-indole-1-carboxylic acid tert-butyl ester
CAS:279255-90-2
Purity:0.96 Package:1g;5g;25g;100g;250g等可分装
|
| Company Name: |
Jinan elephant international trading co., LTD
|
| Tel: |
|
| Email: |
13006575422@163.com |
| Products Intro: |
Product Name:1H-Indole-1-carboxylic acid, 5-(hydroxymethyl)-, 1,1-dimethylethyl ester
CAS:279255-90-2
Purity:98% Package:10g 100g 1kg
|
|
| | 1H-Indole-1-carboxylic acid, 5-(hydroxyMethyl)-, 1,1-diMethylethyl ester Basic information |
| Product Name: | 1H-Indole-1-carboxylic acid, 5-(hydroxyMethyl)-, 1,1-diMethylethyl ester | | Synonyms: | 1H-Indole-1-carboxylic acid, 5-(hydroxyMethyl)-, 1,1-diMethylethyl ester;5-(hydroxymethyl)-1-indolecarboxylic acid tert-butyl ester;5-Hydroxymethyl-indole-1-carboxylic acid tert-butyl ester;5-methylolindole-1-carboxylic acid tert-butyl ester;tert-butyl 5-(hydroxymethyl)indole-1-carboxylate;1-Boc-1H-indol-5-ylmethanol;1H-Indole-1-carboxylic acid, 5-(hydroxyMethyl)-, 1,1-diMethylethyl;(1-Boc-5-indolyl)methanol | | CAS: | 279255-90-2 | | MF: | C14H17NO3 | | MW: | 247.29 | | EINECS: | | | Product Categories: | | | Mol File: | 279255-90-2.mol | 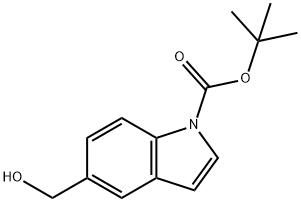 |
| | 1H-Indole-1-carboxylic acid, 5-(hydroxyMethyl)-, 1,1-diMethylethyl ester Chemical Properties |
| Boiling point | 396.0±34.0 °C(Predicted) | | density | 1.14±0.1 g/cm3(Predicted) | | storage temp. | 2-8°C | | pka | 14.37±0.10(Predicted) |
| | 1H-Indole-1-carboxylic acid, 5-(hydroxyMethyl)-, 1,1-diMethylethyl ester Usage And Synthesis |
| Synthesis | General procedure for the synthesis of tert-butyl 5-(hydroxymethyl)-1H-indole-1-carboxylate from tert-butyl 5-formyl-1H-indole-1-carboxylate: firstly, tert-butyl 5-formyl-1H-indole-1-carboxylate (12.23 mmol) was dissolved in methanol (30 mL) to obtain a solution. To this solution, sodium tetrahydroborate (0.463 g, 12.23 mmol) was slowly added at 0 °C. The reaction mixture was stirred continuously at 0 °C for 3 h, followed by stirring at room temperature overnight. After completion of the reaction, the resulting solution was diluted with water (40 mL) and extracted with ethyl acetate (3 x 30 mL). The organic layers were combined, washed sequentially with aqueous sodium carbonate and brine, dried over anhydrous sodium sulfate, and concentrated in vacuum to afford tert-butyl 5-(hydroxymethyl)-1H-indole-1-carboxylate (2.5 g, 10.11 mmol, 83% yield) as a yellow oil.LCMS analysis showed m/z = 270.3 [M + Na]+. | | References | [1] Journal of the American Chemical Society, 2017, vol. 139, # 20, p. 6847 - 6850
[2] Patent: WO2017/216726, 2017, A1. Location in patent: Page/Page column 955 |
| | 1H-Indole-1-carboxylic acid, 5-(hydroxyMethyl)-, 1,1-diMethylethyl ester Preparation Products And Raw materials |
|