|
ChemicalBook Optimization Suppliers |
|
| 化学名: | ウベニメクス | | 英語化学名: | Bestatin | | 别名: | UbenaMix, Inobestin, NK 421;L-Leucine,N-[(2S,3R)-3-aMino-2-hydroxy-1-oxo-4-phenylbutyl]-;N-[(2S,3R)-3-AMino-2-hydroxy-4-phenylbutyryl]-L-leucine 97%;3-(r)-amino-2-(s)-hydroxy-4-phenylbutanoyl-(s)-leucine;n-(3-amino-2-hydroxy-1-oxo-4-phenylbutyl)-,(s-(r*,s*))-l-leucin;nk421;N-[(2S,3R)-3-AMINO-2-HYDROXY-1-OXO-4-PHENYLBUTYL]-L-LEUCINE;N-((2S,3R)-3-AMINO-2-HYDROXY-4-PHENYLBUTYRYL)-L-LEUCINE | | CAS番号: | 58970-76-6 | | 分子式: | C16H24N2O4 | | 分子量: | 308.37 | | EINECS: | 261-529-2 | | カテゴリ情報: | ProteaseInhibitors;Ubenimex;Pepetides;Active Pharmaceutical Ingredients;peptides;Bestatin | | Mol File: | 58970-76-6.mol | 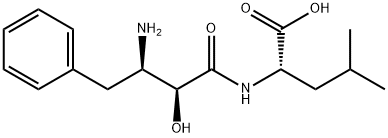 |
| 融点 | 245 °C (dec.)(lit.) | | 比旋光度 | D20 -15.5° (c = 1.0 in 1N HCl) | | 沸点 | 448.76°C (rough estimate) | | 比重(密度) | 1.0917 (rough estimate) | | 屈折率 | 1.5800 (estimate) | | 貯蔵温度 | Keep in dark place,Sealed in dry,Room Temperature | | 溶解性 | Aqueous Acid (Slightly, Sonicated), DMSO (Slightly, Heated) | | 酸解離定数(Pka) | 8.1, 3.1(at 25℃) | | 外見 | Powder | | 色 | White | | 光学活性 (optical activity) | [α]20/D 11°, c = 1 in 1 M NaOH | | 水溶解度 | Soluble in water (<1 25), DMSO (2 mg/ml), methanol (5 mg/ml), 1eq. NaOH (50 mM), ethanol (<1 mg/ml at 25°C), acetic acid, aq. HCl, and alkaline solution. Insoluble in ethyl acetate, benzene, hexane, and chloroform. | | Merck | 13,9910 | | InChIKey | VGGGPCQERPFHOB-RDBSUJKOSA-N | | CAS データベース | 58970-76-6(CAS DataBase Reference) |
| Sフレーズ | 22-24/25 | | WGK Germany | 3 | | RTECS 番号 | OH2915000 | | HSコード | 29242998 | | 毒性 | LD50 in male, female mice, male, female rats (g/kg): 1.3, 1.9, 1.9, 2.1 s.c.; 0.19, 0.19, 0.90, 0.78 i.p.; >4.0, >4.0, >2.0, >2.0 orally (Sakakibara) |
| | ウベニメクス Usage And Synthesis |
| 外観 | 白色~ほとんど白色, 結晶性粉末~粉末 | | 溶解性 | 水、メタノールにはよく溶けやすく、エタノールにはほとんど溶けない。 | | 用途 | 病理研究用 | | 効能 | 抗悪性腫瘍薬, 免疫調節薬 | | 商品名 | ベスタチン (日本化薬) | | 説明 | Bestatin is an aminopeptidase inhibitor originally isolated from S. olivoreticuli. It inhibits aminopeptidase B (IC50 = 0.05 μg/ml), aminopeptidase N (IC50 = 16.9 μM), leucine aminopeptidase (IC50 = 0.01 μg/ml), and the aminopeptidase activity of leukotriene A4 (LTA4) hydrolase (Kapp = 172 nM). It is selective for these aminopeptidases over aminopeptidase A, trypsin, chymotrypsin, elastase, papain, pepsin, and thermolysin. Bestatin inhibits the production of LTB4 in erythrocytes when used at a concentration of 70 μM. It increases the expression of Akt, inhibits proliferation, migration, and invasion, and induces autophagy and apoptosis in 5637 bladder cancer cells. Bestatin (5 and 15 mg/kg) decreases serum levels of LTB4 and reduces tumor growth in a patient-derived xenograft (PDX) mouse model of colorectal cancer. | | 化学的特性 | white powder, soluble in methanol, ethanol, DMSO and other organic solvents, from Streptomyces olivoreticuli. | | Originator | Inst. of Microbial Chem (Japan) | | 使用 | Bestatin is "pseudo"-dipeptide isolated from Strepotmyces olivreticuli in 1976. Bestatin is a potent aminopeptidase B inhibitor with no carboxypeptidase activity. Bestatin acts as an immunomodulator by activating macrophages and T lymphocytes. Bestatin also exhibits good antitumour activity and is has been investigated in clinical trials. | | 使用 | Ubenimex (also known as bestatin) is a competitive aminopeptidase B inhibitor with an IC50 of 100 mg/ml for K562 cells. Proliferation of all the cell lines except KG1 was inhibited by bestatin. P39/TSU, HL60 and U937 were highly sensitive, with 50% growth inhibitory concentration. It is useful in the treatment of non-lymphocytic leukemia. In other types of cancers ubenimex added to radiotherapy or chemotherapy following surgery significantly inmases survival time through immunopotentiation. It is being studied for use in the treatment of acute myelocytic leukemia. | | 主な応用 | Bestatin can indirectly act on monocytes by inhibiting Aminopeptidase B, which in turn inhibits the catabolism of tuftsin. Bestatin has also been shown to inhibit Leukotriene A4 hydrolase. It can directly stimulate lymphocytes through its fixation on the cell surface. The agent has been used in multiple leukemia studies. Bestatin is an aminopeptidase inhibitor, inhibits enkephalin metabolism and leukotriene A4 hydrolase. Inhibits tumor cell proliferation. | | brand name | Bestatin | | 一般的な説明 | Bestatin was found in the culture broth of Streptomyces olivoreticuli in 1976by Umezawa et al. in the course of screening specific enzyme inhibitors of microbial origin. It shows inhibitory activity against specific exopeptidases, e.g., aminopeptidase B and leucine aminopeptidase. However, it does not act against aminopeptidase A, carboxypeptidases A and B, or endopeptidases. Bestatin acts as a bioresponse modifier and shows antitumor activity via stimulation of immuno response in the host. In combination with cytotoxic anticancer drugs, it is now under clinical evaluation for use in cancer therapy. | | 生物活性 | Aminopeptidase inhibitor (K i = 0.001-90 mM); inhibits enkephalin metabolism and leukotriene A 4 hydrolase. Inhibits tumor cell proliferation. | | 安全性プロファイル | Poison by intraperitoneal route.Moderately toxic by subcutaneous route. Experimentalreproductive effects. When heated to decomposition itemits toxic fumes of NOx. | | 貯蔵 | Store at +4°C | | Mode of action | Bestatin acts as an immunomodulator by activating macrophages and T lymphocytes. | | Clinical claims and research | Bestatin (ubenimex) is a potent inhibitor of aminopeptidase N and aminopeptidase B, which was isolated from a culture filtrate of Streptomyces olivoreticuli during the search for specific inhibitors of enzymes present on the membrane of eukaryotic cells. Inhibitors of aminopeptidase activity are associated with macrophage activation and differentiation, and Bestatin has shown significant therapeutic effects in several clinical trials. In one multi-institutional study, patients with acute non-lymphocytic leukemia (ANLL) were randomized to receive either Bestatin or control orally after completion of induction and consolidation therapy, and concomitant with maintenance chemotherapy. Remission duration was prolonged in the Bestatin group, although this difference did not reach statistical significance. However, OS was prolonged in the Bestatin group. Recently, a confirmatory phase III trial in ANLL was reported that extended the observation to a significant prolongation of remission. Bestatin has also shown adjuvant activity when administered to acute leukemia and CML patients who did not develop graft-versus-host disease (GVHD) within 30 days following BMT. Bestatin-treated acute leukemia patients had an increased incidence of chronic low-grade GVHD compared with the control arm and a lower relapse rate. Recently, a phase III study of resected stage 1 squamous cell lung cancer patients treated orally with either Bestatin or placebo daily for 2 years revealed that 5-year cancer-free survival was significantly greater in the Bestatin group (71%) as compared to the placebo group (62%). OS was also significantly improved, as was cancer-free survival. Recent studies in patients with nonsmall cell lung cancer (NSCLCs) suggest that it also has anti-angiogenic activity. | | 参考文献 | 1. umezawa h, aoyagi t, suda h, hamada m, takeuchi t, bestatin, an inhibitor of aminopeptidase b, produced by actinomycetes, j antibiot (tokyo). 1976 jan; 29(1):97-9.2. umezawa, h., aoyagi, t., suda, h., hamada, m., and takeuchi, t. 197615. antibiotics 29, 97.3. suda, h., takita, t., aoyagi, t., and umezawa, h. (1976) j. antibiotics 29, 100.4. nakamura, h., suda, h., takita, t., aoyagi, t., umezawa, h., and iitaka, y. (1976) j. antibiotics 29, 102.5. umezawa, s., tsuchiya, t., and tatsuta, k. (1966) bull. chem. sot. japan 39, 1235. 6. barlow, c. b., and gijthrie, r. d. (1967) j. chem. sot. (c) 1194. 7. bukhari, s. t. k., guthrie, r. d., scott, a. i., and wrixon, a. d. (1970) tetrahedron 26, 3653.8. suda et al. inhibition of aminopeptidase b and leucine aminopeptidase by bestatin and its stereoisomer, archives of biochemistry and biophysics, 77, 196-200 (1976) |
|