|
ChemicalBook Optimization Suppliers |
|
| 化学名: | バイアグラ | | 英語化学名: | Sildenafil citrate | | 别名: | 1-[[3-(4,7-Dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo[4,3-d]pyrimidin-5-yl)-4-ethoxyphenyl]sulfonyl]-4-methylpiperazine citrate salt;1-[[3-(6,7-Dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo[4,3-d]pyrimidin-5-yl)-4-ethoxyphenyl]sulfonyl]-4-methylpiperazine, 2-hydroxy-1,2,3-propanetricarboxylate;Sildenafil Citrate (100 mg);Sildenafil citrate, >=99%;Sildenafil citrate, Professional supply;5-[2-Ethoxy-5-[(4-methyl-piperazin-1-yl)sulfonyl]phenyl]-1,6-dihydro-1-methyl-3-propyl-7H-pyrazolo[4,3-d]pyrimidin-7-one citrate;5-(2-Ethoxy-5-((4-methylpiperazin-1-yl)sulfonyl)phenyl)-1-methyl-3-propyl-1H-pyrazolo[4,3-d]py;SILDENAFIL CITRATE PH.EUR | | CAS番号: | 171599-83-0 | | 分子式: | C28H38N6O11S | | 分子量: | 666.7 | | EINECS: | 200-659-6 | | カテゴリ情報: | Inhibitors;Intermediates & Fine Chemicals;Pharmaceuticals;Sildenafil;Pfizer compounds;Inhibitor;organic interme;171599-83-0 | | Mol File: | 171599-83-0.mol | 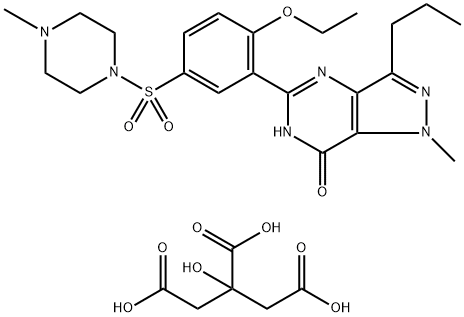 |
| 融点 | 187-189°C | | 貯蔵温度 | 2-8°C | | 溶解性 | DMSO: >20mg/mL | | 外見 | white powder | | 色 | White | | 水溶解度 | 3.488g/L(temperature not stated) | | Merck | 14,8489 | | BCS Class | 1 | | 安定性: | Store in Freezer | | InChIKey | DEIYFTQMQPDXOT-UHFFFAOYSA-N | | SMILES | C1(=CC(=CC=C1OCC)S(=O)(=O)N1CCN(C)CC1)C1=NC(C2N(N=C(CCC)C=2N1)C)=O.C(O)(C(=O)O)(CC(=O)O)CC(=O)O | | CAS データベース | 171599-83-0(CAS DataBase Reference) |
| 主な危険性 | Xi | | Rフレーズ | 36/37/38 | | Sフレーズ | 36 | | WGK Germany | 3 | | RTECS 番号 | TL4284390 | | HSコード | 2933595960 |
| | バイアグラ Usage And Synthesis |
| 外観 | 白色~わずかにうすい褐色、結晶~粉末 | | 溶解性 | 水及びメタノールに溶けにくく、エタノール(95)にほとんど溶けない。 | | 解説 | バイアグラ.ホスホジエステラーゼの阻害剤で,インポテンツの改善薬として使われている.アメリカのファイザー社が1998年発売した性的不能治療薬の商品名。3か月で300億円と史上最高の売上げを記録、わずか1年足らずで約50か国で認可された。もともとは心臓病薬として開発されたが、男性のペニスの勃起(ぼっき)作用が注目され、生活改善薬に転換した。バイアグラは、ペニスの血流をおさえる酵素のはたらきをおさえ、ペニスの血管を拡張する。しかし勃起効果の半面、ニトログリセリンなど心臓病薬との併用者が死亡するなど副作用による事故もおこっており、問題視する声もある。 | | 用途 | 強力なホスホジエステラーゼ 5(PDE5)阻害剤です(IC50=4 nM)。経口活性があります。 | | 用途 | 強力なホスホジエステラーゼ 5(PDE5)阻害剤です(IC50=4 nmol/l)。経口活性があります。 | | 用途 | ホスホジエステラーゼ5 (PDE-5)酵素活性阻害作用を示します。 | | 用途 | ホスホジエステラーゼ5
(PDE-5)酵素活性阻害作用を示します。 | | 効能 | 血管拡張薬, 勃起不全治療薬, ホスホジエステラーゼV阻害薬 | | 商品名 | シルデナフィル (あすか製薬); シルデナフィル (キッセイ薬品工業); シルデナフィル (シオノケミカル); シルデナフィル (大興製薬); シルデナフィル (大興製薬); シルデナフィル (大興製薬); シルデナフィル (富士化学工業); シルデナフィル (東和薬品); シルデナフィル (武田テバファーマ); シルデナフィル (辰巳化学); シルデナフィル (陽進堂); バイアグラ (ファイザー); レバチオ (ファイザー) | | 使用上の注意 | アルゴン封入 | | 説明 | Sildenafil is a potent inhibitor of phosphodiesterase 5 (PDE5) with IC50 values of 3.6 and 3 nM for PDE5 activity in isolated rabbit platelets and human corpus cavernosum, respectively. It is selective for PDE5 over PDE1 and PDE3 (IC50s = 0.26 and 65 μM, respectively). Sildenafil reverses glucose-induced decreases in angiopoietin 1 (ANG1) expression and reduction of capillary-like tube formation by mouse dermal endothelial cells in vitro and increases the number of functional blood vessels and regional blood flow in the sciatic nerve in a db/db mouse model of diabetic peripheral neuropathy. It increases the ratio of maximum intracavernosal pressure to mean arterial blood pressure (ICP/MAP), a measure of erectile function, in castrated rats when administered at a dose of 20 mg/kg per day. Sildenafil (0.5 mg/kg) also reduces cardiac arrest and resuscitation-induced increases in angiotensin II , angiotensin converting enzyme (ACE), ACE2, and various angiotensin receptors and increases survival in a porcine model of ischemia/reperfusion injury. Formulations containing sildenafil have been used in the treatment of erectile dysfunction, pulmonary arterial hypertension, and high-altitude pulmonary edema associated with altitude sickness. | | 化学的特性 | Sildenafil citrate is a white to off-white crystalline powder soluble in DMF, acetic acid and slightly soluble in methanol. Solubility of sildenafil citrate is pH dependent and it decreases with increase of pH. pH ranges between 3.7 and 3.8 and the pKa from 8.2 to 9.6. | | Originator | Alsigra,Alembic Ltd.,India | | 使用 | Sildenafil citrate is an orally active selective type 5 cgmp phosphodiesterase inhibitor that is used in the treatment of erectile dysfunction and primary pulmonary hypertension. | | 定義 | ChEBI: Sildenafil citrate is the citrate salt of sildenafil. It has a role as a vasodilator agent and an EC 3.1.4.35 (3',5'-cyclic-GMP phosphodiesterase) inhibitor. It contains a sildenafil. | | Manufacturing Process | 4-(2-Ethoxybenzamido)-1-methyl-3-n-propylpyrazole-5-carboxamide (223 g, 0.676 mol) was added portionwise to a solution of sodium hydroxide (54 g, 1.35 mol) and 30% hydrogen peroxide solution (224 ml) in water (2000 ml). Ethanol (700 ml) was added and the resulting mixture heated under reflux for 2.5 h, cooled, then evaporated under vacuum. The resulting solid was treated with 2 N hydrochloric acid (380 ml), with external cooling, and the mixture was extracted with dichloromethane (1 x 700 ml, 3 x 200 ml). The combined organic extracts were washed successively with saturated aqueous sodium carbonate solution (3 x 400 ml) and brine (300 ml), then dried (Na2SO4) and evaporated under vacuum. Chromatography of the residue on silica gel (1000 g), using a methanol in dichloromethane elution gradient (0-1%), followed by trituration of the crude product with ether (300 ml), gave the 5-(2- ethoxyphenyl)-1-methyl-3-n-propyl-l,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin- 7-one as a colourless solid (152.2 g, 72%), melting point 143°-146°C.
5-(2-Ethoxyphenyl)-1-methyl-3-n-propyl-l,6-dihydro-7H-pyrazolo[4,3- d]pyrimidin-7-one (10.0 g, 32.1 mmol) was added portionwise to chlorosulfonic acid (20 ml) at 0°C under a nitrogen atmosphere. After being stirred overnight, the reaction solution was cautiously added to ice-water (150ml) and the aqueous mixture extracted with a 9:1 mixture of dichloromethane and methanol (4 x 100 ml). The combined extracts were dried (Na2SO4) and evaporated under vacuum to give the required 5-(5-chlorosulphonyl-2- ethoxyphenyl)-1-methyl-3-n-propyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin- 7-one as a white solid (12.8 g, 97%), melting point 179°-181°C.
4-Methylpiperidine was added to a stirred suspension of 5-(5-chlorosulphonyl- 2-ethoxyphenyl)-1-methyl-3-n-propyl-1,6-dihydro-7H-pyrazolo[4,3- d]pyrimidin-7-one in ethanol at room temperature. The resulting mixture was stirred for 4 days before removing the solvent by evaporation under vacuum. The residue was dissolved in a 9:1 mixture of dichloromethane and methanol and the solution washed with saturated aqueous sodium carbonate solution. The aqueous phase was further extracted with dichloromethane-methanol mixtures (3 x 100 ml) and all the organic fractions were combined, dried (MgSO4) and evaporated under vacuum to give a solid. Crystallisation from a mixture of methanol-dimethylformamide gave the 5-[2-ethoxy-5-(4- methylpiperidinylsulphonyl)phenyl]-1-methyl-3-n-propyl-1,6-dihydro-7Hpyrazolo[ 4,3-d]-pyrimidin-7-one as an off-white solid, melting point 187°- 189°C.
After addition of citric acid to the 5-[2-ethoxy-5-(4- methylpiperidinylsulphonyl)phenyl]-1-methyl-3-n-propyl-1,6-dihydro-7Hpyrazolo[ 4,3-d]-pyrimidin-7-one (sildenafil) the it's salt is obtained, namely sildenafil citrate. | | brand name | Viagra (Pfizer). | | Therapeutic Function | Vasodilator | | 一般的な説明 | Sildenafil Citrate is the citrate salt form of sildenafil, an orally bioavailable pyrazolopyrimidinone derivative structurally related to zaprinast, with vasodilating and potential anti-inflammatory activities. Upon oral administration, sildenafil selectively targets and inhibits cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5), thereby inhibiting the PDE5-mediated degradation of cGMP found in smooth muscle and increasing cGMP availability. This results in prolonged smooth muscle relaxation in the corpus cavernosum of the penis, thereby causing vasodilation, blood engorgement and a prolonged penile erection. In the smooth muscle of the pulmonary vasculature, the increase in cGMP results in smooth muscle relaxation, vasodilation of the pulmonary vascular bed, relieving pulmonary hypertension and increasing blood flow in the lungs. In addition, sildenafil may reduce airway inflammation and mucus production. | | 生物活性 | Orally active, potent inhibitor of phosphodiesterase 5 (PDE5) (IC 50 = 4 nM). Enhances nitric oxide-dependent relaxation of human corpus cavernosum in vitro . | | Biochem/physiol Actions | Sildenafil is a potent, selective inhibitor of cGMP specific phosphodiesterase type 5 (PDE5). Sildenafil is used to treat erectile dysfunction and pulmonary arterial hypertension. NO activates guanylate cyclase, which results in increased levels of cGMP, producing smooth muscle relaxation. Sildenafil enhances the effect of NO by inhibiting PDE5, which is responsible for degradation of cGMP. | | 薬物動態学 | Analysis of Middle Eastern data (mean ± SD) revealed Cmax = 398.9 ± 107.7 ng/ml; Tmax = 1.84 ± 0.22 h; t1/2 = 2.66 ± 0.97 h; AUC0–24 = 1475 ± 515.3 ng.h/ml; AUC0-∞ = 1556 ± 567.58 ng.h/ml. | | 副作用 | The more common side effects of sildenafil citrate include: headache, skin flushing, indigestion, abnormal vision, nasal congestion, back pain, nausea, dizziness, rash, etc. | | 安全性プロファイル | A poison by ingestion. Human systemic effects. When heated to decomposition it emits toxic vapors of NOx and SOx. | | 合成 | The synthesis of sildenafil citrate was first reported in the Bioorganic & Medicinal Chemistry Letters, Vol 6, pp. 1819, 1824, 1996. The reaction scheme is reproduced below. Sildenafil was reported in this journal as "a potent and selective inhibitor of type 5 PDE with utility for the treatment of male erectile dysfunction".
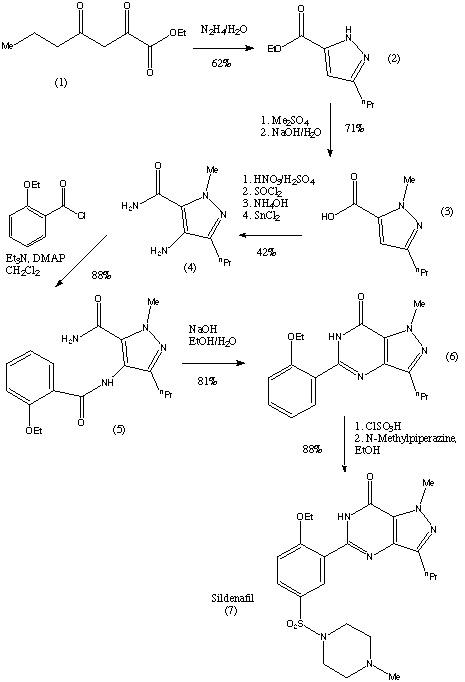
The first step of the synthesis is the reaction of a diketoester (1) and hydrazine to give the pyrazole ring. The regioselective N-methylation of the pyrazole and hydrolysis gives a carboxylic acid (3). Compound (3) is then reacted with HNO3 and H2SO4 to give a nitrated product.
This is then followed by a carboxamide formation and the reduction of the nitro group. The compound (4) is then acylated under basic conditions and this produces the pyrazolopyrimidinone (6). (6) is then chlorosulphonylated selectively on the 5'-position of the phenyl ring. This can then couple with an amine to give sildenafil (7).
www.ch.ic.ac.uk/local/projects/p_hazel/synthesis2.html | | Veterinary Drugs and Treatments | Sildenafil may be of benefit in the adjunctive treatment of pulmonary
hypertension in small animals.
In humans, sildenafil is indicated for erectile dysfunction or pulmonary
hypertension. | | 貯蔵 | Desiccate at RT | | structure and hydrogen bonding | Sildenafil citrate (SC) has been widely used for the treatment of erectile disorder. A detailed study concerning solid-state structure of this compound is very important for understanding enzyme (PDE5)-inhibitor (sildenafil) interaction. It is also of interest to determine sildenafil’s protonation sites, as they may be responsible for its binding to the phosphodiesterase acidic amino acids.
Sildenafil citrate (Viagra) and sildenafil base in pure form were characterized by 1H, 13C, 15N NMR spectroscopy in solution, solid-state, and pharmaceutical dosage forms.42 The analysis of chemical shifts showed that: (i) N6-H forms intramolecular hydrogen bonds, (ii) N25 is protonated in the salt, and (iii) intermolecular OH. . .N hydrogen bonds involving N2 and N4 are present in the solid sildenafil citrate. The 13C CPMAS spectra of the tablets containing different amounts of sildenafil citrate were recorded and showed that chemical shifts of sildenafil citrate in pure form and in pharmaceutical dosage forms are the same. SC is easily detected in the pharmaceutical dosage forms since only two of its carbon resonances (OCH2 and quaternary carbon of the citrate anion) fall into carbohydrate-type region of the excipient.
Solid-state 13C and 15N MAS NMR have recently been used to investigate how water interacts with SC.43 When the humidity is altered, the water concentration in the solid compound changes but does not reach a stoichiometric (e.g., 1:1) ratio to form a true hydrate. Only one set of 15N and 13C signals was observed for each humidity level indicating that water incorporated into the crystal lattice of SC is very mobile and exchanges rapidly between various sites. The 13C data showed the formation of a hydrogen bond between water molecule and one carbonyl of the citrate anion. The spectra also show that the water content affects the conformation of the propyl group. Additionally, 15N dipolar dephasing (DD) experiments confirmed that the sildenafil molecule is only protonated in the piperazine ring. |
|