|
ChemicalBook Optimization Suppliers |
| 名前: |
Alfa Aesar |
| 電話番号: |
400-6106006 |
| 電子メール: |
saleschina@alfa-asia.com |
| 名前: |
Energy Chemical |
| 電話番号: |
021-021-58432009 400-005-6266 |
| 電子メール: |
sales8178@energy-chemical.com |
|
| 化学名: | (+)-ウスニン酸 | | 英語化学名: | (+)-Usniacin | | 别名: | D-2,6-DIACETYL-7,9-DIHYDROXY-8,9BETA-DIMETHYL-1,3[2H,9BH]-DIBENZOFURANDIONE;D-USNIC ACID;TIMTEC-BB SBB006458;(+)-USNEIN;(+)-USNIACIN;(r)-usnicaci;3(2h,9bh)-dibenzofurandione,2,6-diacetyl-7,9-dihydroxy-8,9b-dimethyl-(9b;6-diacetyl-1,7,9-trihydroxy-8,9b-dimethyl-d-3(9bh)-dibenzofuranon | | CAS番号: | 7562-61-0 | | 分子式: | C18H16O7 | | 分子量: | 344.32 | | EINECS: | 231-456-0 | | カテゴリ情報: | Inhibitors;Pharmaceutical Raw Materials;Plant Oils, Toxins, Phenolic Acids & Derivatives | | Mol File: | 7562-61-0.mol | 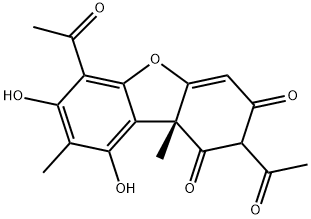 |
| 融点 | 201-203 °C(lit.) | | 比旋光度 | 488 º (c=0.4, CHCl3) | | 沸点 | 399.43°C (rough estimate) | | 比重(密度) | 1.2869 (rough estimate) | | 屈折率 | 1.4790 (estimate) | | 貯蔵温度 | Sealed in dry,Room Temperature | | 溶解性 | almost transparency in Chloroform | | 外見 | powder to crystal | | 酸解離定数(Pka) | 4.00±0.40(Predicted) | | 色 | Light orange to Yellow to Green | | 光学活性 (optical activity) | [α]25/D +488°, c = 0.7% in chloroform | | 水溶解度 | Partly soluble in water. Soluble in acetone, ethanol, chloroform and furfural. | | Merck | 14,9893 | | BRN | 96698 | | LogP | 1.270 (est) | | CAS データベース | 7562-61-0(CAS DataBase Reference) |
| 主な危険性 | Xn | | Rフレーズ | 22 | | Sフレーズ | 36 | | WGK Germany | 3 | | RTECS 番号 | HP5295050 | | F | 3-10 | | 国連危険物分類 | IRRITANT | | HSコード | 29329990 |
| | (+)-ウスニン酸 Usage And Synthesis |
| 外観 | 黄色~暗黄色, 結晶~結晶性粉末 | | 溶解性 | 水に不溶。エタノール, ベンジンに難溶。クロロホルム, ベンゼンに易溶。水に溶けない。エタノール、ベンジンに溶けにくく、クロロホルム、ベンゼンに溶けやすい。クロロホルムに溶け、エタノールに極めて溶けにくく、水にほとんど溶けない。 | | 特長 | 生物学的性質 : 試験管内においてグラム陽性菌、結核菌などに対して成長抑制作用がある。 | | 使用 | (+)-Usnic acid has been used to study the following:
- The mechanism of its antimicrobial activity in bacterial cells.
- Its ability as an antibiofilm agent against Group A Streptococci (GAS).
- Its ability as a potent anti-virulent compound against Candida albicans.
- The mechanism of its toxic effect on hepatocytes.
- Its ability to inhibit the motility of human lung cancer cells.
| | 使用 | Usnic Acid acts as an antibacterial and antifungal agent, and are often seen used in cosmetics and facial application chemicals. | | 使用 | Usnic acid is used as fragrance; preservative in deodorants; in anti ac ne formulations; antibiotic for topical application. | | 定義 | ChEBI: (-)-usnic acid is the (-)-enantiomer of usnic acid. It has a role as an EC 1.13.11.27 (4-hydroxyphenylpyruvate dioxygenase) inhibitor. It is a conjugate acid of a (-)-usnic acid(2-). It is an enantiomer of a (+)-usnic acid. | | 一般的な説明 | (+)-Usniacin is a primary reference substance with assigned absolute purity (considering chromatographic purity, water, residual solvents, inorganic impurities). The exact value can be found on the certificate. Produced by PhytoLab GmbH & Co. KG. | | 生物活性 | mic: 0.05 μg/62.5 μl to 3.1 μg/62.5 μlmicroorganisms can colonize a wide variety of medical devices, putting patients at risk for systemic and local infectious complications, including local-site infections, endocarditis, and catheter-related bloodstream infections. (+)-usniacin is a secondary lichen metabolite that possesses antimicrobial activity against various planktonic gram-positive bacteria. | | 接触アレルゲン | Usnic acid is a component of lichens, also used as a
topical antibiotic. Allergic contact dermatitis from
lichens occurs mainly occupationally in forestry and
horticultural workers, and in lichen pickers. | | in vitro | (+)-usniacin showed antimicrobial activity against the same microorganisms as that of acetone extract. among the three analogues it was the most active one having quite low mic values. furthermore, (+)-usniacin did not show any activity against a. hydrophila and b. cereus whereas (d)-usnic acid did. on the other hand, (+)-usniacin was active against y. enterocolitica whereas (d)-usnic acid was not active [1]. | | 純化方法 | This very weak acid is the natural form which is recrystallised from Me2CO, MeOH or *C6H6. At 25o it is soluble in H2O (<0.01%), Me2CO (0.77%), EtOAc (0.88%), MeOCH2CH2OH (0.22%) and furfural (7.32%). [Curd & Robertson J Chem Soc 894 1937, Barton & Brunn J Chem Soc 603 1953, resolution: Dean et al. J Chem Soc 1250 1953, synthesis: Barton et al. J Chem Soc 538 1956, Beilstein 18/5 V 586.] | | 参考文献 | [1] tay t, türk ao, yilmaz m, türk h, kivanç m. evaluation of the antimicrobial activity of the acetone extract of the lichen ramalina farinacea and its (+)-usnic acid, norstictic acid, and protocetraric acid constituents. z naturforsch c. 2004 may-jun;59(5-6):384-8.
[2] ghione m, parrello d, grasso l. usnic acid revisited, its activity on oral flora. chemioterapia. 1988 oct;7(5):302-5. |
|