|
ChemicalBook Optimization Suppliers |
|
| 化学名: | フィナステリド | | 英語化学名: | Finasteride | | 别名: | (5alpha,17beta)-(1,1-dimethylethyl)-3-oxo-4-azaandrost-1-ene-17-carboxamide;17beta-(n-tert-butylcarbamoyl)-4-aza-5alpha-androst-1-en-3-one;4-azaandrost-1-ene-17-carboxamide,n-(1,1-dimethylethyl)-3-oxo-,(5-alpha,17-be;4-azaandrost-1-ene-17-carboxamide,n-(1,1-dimethylethyl)-3-oxo-,(5alpha,17beta;l-652,931;mk-0906;(5alpha,17beta)-N-(1,1-Dimethylethyl)-3-oxo-4-azaandrost-1-ene-17-carboxamide;(5α,17β)-N-(1,1-Dimethylethyl)-3-oxo-4-azaandrost-1-ene-17-carboxamide | | CAS番号: | 98319-26-7 | | 分子式: | C23H36N2O2 | | 分子量: | 372.54 | | EINECS: | 620-534-3 | | カテゴリ情報: | Hormone Drugs;Biochemistry;Steroids;Steroids (Others);Vitamin Ingredients;Intermediates & Fine Chemicals;Pharmaceuticals;Active Pharmaceutical Ingredients;API;Steroid and Hormone;Intracellular receptor;PROSCAR;98319-26-7 | | Mol File: | 98319-26-7.mol | 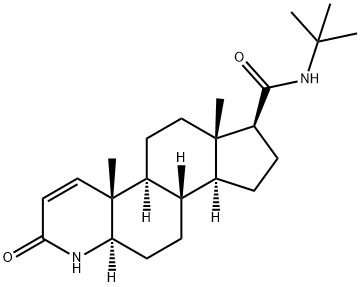 |
| 融点 | 253 °C | | 比旋光度 | 405 -59° (c = 1 in methanol) | | 沸点 | 576.6±50.0 °C(Predicted) | | 比重(密度) | 1.065±0.06 g/cm3(Predicted) | | 貯蔵温度 | room temp | | 溶解性 | DMSO: 32 mg/mL, soluble | | 外見 | solid | | 酸解離定数(Pka) | 14.17±0.70(Predicted) | | 色 | white to beige | | 水溶解度 | insoluble | | Merck | 14,4082 | | BCS Class | 1 | | InChIKey | DBEPLOCGEIEOCV-WSBQPABSSA-N | | LogP | 3.030 | | CAS データベース | 98319-26-7(CAS DataBase Reference) | | EPAの化学物質情報 | 1H-Indeno[5,4-f]quinoline-7-carboxamide, N-(1,1-dimethylethyl)-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-4a,6a-dimethyl-2-oxo-, (4aR,4bS,6aS,7S,9aS,9bS,11aR)- (98319-26-7) |
| | フィナステリド Usage And Synthesis |
| 外観 | 白色~ほとんど白色粉末~結晶 | | 用途 | フィナステリド(finasteride)は、アメリカメルク社が開発した抗アンドロゲン薬。2型5-α還元酵素を阻害して、男性ホルモンテストステロンがDHT(ジヒドロテストステロン)に転換されるのを抑制する。高用量(5mg/day)で前立腺肥大症?前立腺癌に対して抑制的に作用する。Proscar等の商品名で海外で販売されているが、日本では前立腺の治療薬としては未承認。
低用量(0.2または1mg/day)で、男性型脱毛症(AGA)に対して脱毛抑制効果を認め、プロペシア(Propecia)の商品名で多くの国で発売されている。 | | 効能 | 抗アンドロゲン薬, 5α還元酵素阻害薬 | | 商品名 | フィナステリド (シオノケミカル); フィナステリド (ファイザー); フィナステリド (リョートーファイン); フィナステリド (大興製薬); フィナステリド (富士化学工業); フィナステリド (小林化工); フィナステリド (東和薬品); フィナステリド (武田テバファーマ); フィナステリド (沢井製薬); フィナステリド (辰巳化学); フィナステリド (辰巳化学); プロペシア (MSD) | | 説明 | Finasteride, a novel 4-azasteroid, is a breakthrough in the treatment and control of
benign prostatic hyperplasia. Mechanistically, it inhibits the prostatic-specific enzyme
5-alpha reductase, thereby decreasing the conversion of testosterone to
dihydrotestosterone. It is reportedly effective in reducing urinary symptoms and
prostatic volume and increasing maximal urinary flow rate. Finasteride is also being
investigated as a treatment for prostatic cancer. | | 化学的特性 | White or off-white crystalline powder. Soluble in chloroform, dimethyl sulfoxide, ethanol, methanol, or n-propanol. Insoluble in propylene glycol or polyethylene glycol 400. Very slightly soluble in 0.1mol/L hydrochloric acid, 0.1mol/L sodium hydroxide solution, or water. | | Originator | Merck (U.S.A.) | | 使用 | Finasteride is an antialopecia agent that Inhibitor of 5α-reductase, the enzyme which converts testosterone to the more potent androgen, 5α-dihydrotestosterone. It is used to treatment of benign prostatic hyperplasia and androgenetic alopecia. | | 定義 | ChEBI: Finasteride is an aza-steroid that is a synthetic drug for the treatment of benign prostatic hyperplasia. It has a role as an androgen antagonist, an EC 1.3.1.22 [3-oxo-5alpha-steroid 4-dehydrogenase (NADP(+))] inhibitor and an antihyperplasia drug. It is an aza-steroid, a 3-oxo steroid and a delta-lactam. It derives from a hydride of a 5alpha-androstane. | | 主な応用 | Finasteride is a specific inhibitor of steroid type II 5α-reductase, an intracellular enzyme that converts testosterone to DHT. By inhibiting type II 5α-reductase, this conversion is blocked, resulting in significant decreases in serum and tissue DHT concentrations. Merck & Co. developed finasteride as an oral treatment for androgenetic alopecia after men taking finasteride (5 mg/ day) for prostate enlargement noticed regrowth of their hair. Finasteride is indicated for use in men only. Women of childbearing age cannot take finasteride because it may cause hypospadia (a developmental abnormality of the penis) in the male offspring if taken during pregnancy. | | 適応症 | Finasteride (Proscar) is a 5�-reductase inhibitor that
blocks the conversion of testosterone to DHT in target
tissues. Since DHT is the major intracellular androgen
in the prostate, finasteride is effective in suppressing
DHT stimulation of prostatic growth and secretory
function without markedly affecting libido. It is approved
for the treatment of benign prostatic hyperplasia.
Although there is usually some regression in the size
of the prostate gland following administration of finasteride,
clinical response may take 6 to 12 months. If the
obstructive symptoms are severe, there is often not
enough time to allow this compound to work.The principal
adverse effects of finasteride are impotence, decreased
libido, and decreased volume of ejaculate. The
compound is generally well tolerated in men. | | Manufacturing Process | In a flask equipped with an overhead stirrer, a nitrogen inlet, and reflux
condenser was placed 840 ml of dry THF and 20.0 g of 17β-carboxylate 17β-
carbomethoxy ester of 4-aza-5α-androst-1-en-3-one (synthesized according to Patent US 4,377,584, issued Mar. 22,1883, and J. Med. Chem., 29, 2298
(1986)). The resulting slurry was cooled to -5-10°C, and 27.6 mL of tbutylamine
was added. A solution of ethylmagnesium bromide in THF (122
mL, 2 M) was added maintaining the temperature of the reaction mixture
below 10°C. The reaction mixture was heated at reflux for 12 hours and was
added to a cold (10°C) solution of 25% ammonium chloride in water. The
mixture was warmed to 25°C and allowed to settle. The THF solution was
separated and concentrated by atmospheric distillation to 200 mL and the
product was crystallized by adding approximately 600 mL of dilute aqueous
HCl. The resulting white solid was isolated by filtration and was dried at 70°C
under vacuum to give 21.7 g (97% yield) 2-butyl-1-(4-carboxybenzyl)-4-
chloroimidazole-5-acetic acid of finasteride. The finasteride can be purified by
conventional procedures, e.g. recrystallization from methylene chloride/ethyl
acetate or acetic acid/water, melting point 261°C. | | brand name | Proscar | | Therapeutic Function | Antineoplastic | | 生物活性 | Antiandrogen that inhibits type II 5 α reductase (IC 50 = 65 nM). Suppresses the conversion of testosterone to dihydrotestosterone. Reduces prostatic dihydrotestosterone levels and prostate size in vivo . Orally active. | | Biochem/physiol Actions | Selective 5α-reductase inhibitor; antiandrogen. | | 薬物動態学 | The mean oral bioavailability of finasteride is 65%, as shown in Table 45.4, and is not affected by food. Approximately 90% of circulating finasteride is bound to plasma proteins. Finasteride has been found to cross the blood-brain barrier, but levels in semen were undetectable (<0.2 ng/mL). Finasteride is extensively metabolized in the liver, primarily via CYP3A4 to two major metabolites: monohydroxylation of the t-butyl side chain, which is further metabolized via an aldehyde intermediate to the second metabolite, a monocarboxylic acid. The metabolites show approximately 20% the inhibition of finasteride for 5α-reductase. The mean terminal half-life is approximately 5 to 6 hours in men between 18 and 60 years of age and 8 hours in men older than 70 years of age. Following an oral dose of finasteride, approximately 40% of the dose was excreted in the urine as metabolites and approximately 57% in the feces. Even though the elimination rate of finasteride is decreased in the elderly, no dosage adjustment is necessary. No dosage adjustment is necessary in patients with renal insufficiency. A decrease in the urinary excretion of metabolites was observed in patients with renal impairment, but this was compensated for by an increase in fecal excretion of metabolites. Caution should be used during administration to patients with liver function abnormalities, because finasteride is metabolized extensively in the liver. | | 臨床応用 | The selective inhibition of the type 2 5α-reductase isozyme produces a rapid reduction in plasma
DHT concentration, reaching 65% suppression within 24 hours of administering a 1-mg oral tablet
(106). At steady state, finasteride suppresses DHT levels by approximately 70% in plasma and by as
much as 85 to 90% in the prostate. The remaining DHT in the prostate likely is the result of type 1
5α-reductase. The mean circulating levels of testosterone and estradiol remained within their physiological concentration range. Long-term
therapy with finasteride can reduce clinical significant end points of BPH, such as acute urinary
retention or surgery. Finasteride is most effective in men with large prostates. Finasteride has no
affinity for the AR and no androgenic, antiandrogenic, estrogenic, antiestrogenic, or progestational
effects. | | Veterinary Drugs and Treatments | Finasteride may be useful in treating the benign prostatic hypertrophy
in canine patients. Because of the drug’s relative expense and
the long duration of therapy required to see a response, its usefulness
may be limited in veterinary medicine.
It may also be useful in the adjunctive treatment of adrenal disease
in ferrets. | | 代謝 | Finasteride is metabolised primarily via the cytochrome
P450 3A4 enzyme subfamily. Following an oral dose
of 14C-finasteride in man, two metabolites of the drug
were identified that possess only a small fraction of the
5α-reductase inhibitory activity of finasteride. 39% of the
dose was excreted in the urine in the form of metabolites
(virtually no unchanged drug was excreted in the urine)
and 57% of total dose was excreted in the faeces. | | 貯蔵 | Store at RT | | 参考文献 | Perifollicular fibrosis: Pathogenetic role in androgenetic alopecia; H.G. Yoo, et al.; Biol. Pharm. Bull. 29, 1246 (2006). DOI:10.1248/BPB.29.1246
Finasteride induces apoptosis via Bcl-2, Bcl-xL, Bax and caspase-3 proteins in LNCaP human prostate cancer cell line: J.M. Golbano, et al.; Int. J. Oncol. 32, 919 (2008) DOI:10.3892/IJO.32.4.919
Steroid 5α-reductase as a novel therapeutic target for schizophrenia and other neuropsychiatric disorders: S. Paba, et al.; Curr. Pharm. Des. 17, 151 (2011) DOI:10.2174/138161211795049589
Finasteride: An update of its use in the management of symptomatic benign prostatic hyperplasia (a review). M. I. Wilde, K. L. Goa, Drugs 1999, 57, 557. DOI:10.2165/00003495-199957040-00008
Finasteride: a review of its use in male pattern hair loss. K. J. McClellan, A. Markham, Drugs 1999, 57, 111. DOI:10.2165/00003495-199957010-00014
Type 1 and type 2 5α-reductase expression in the development and progression of prostate cancer (a review). L. N. Thomas, R. C. Douglas, C. B. Lazier, C. K. L. Too, R. S. Rittmaster, D. J. Tindall, Eur. Urol. 2008, 53, 244. DOI:10.1016/J.EURURO.2007.10.052 |
|