|
ChemicalBook Optimization Suppliers |
|
| 化学名: | こはく酸 | | 英語化学名: | Succinic acid | | 别名: | Succinic acid, 99% 250GR;Succinic acid, 99% 500GR;SUCCINIC ACID 99+% FCC;SUCCINIC ACID, 99+%;SUCCINIC ACID, MATRIX SUBSTANCE FOR MALD I-MS;SUCCINIC ACID, PGE. WITH 10 X 10 MG;SUCCINIC ACID, REAGENTPLUS TM, >= 99.0%;SUCCINIC ACID FREE ACID ACS REAGENT | | CAS番号: | 110-15-6 | | 分子式: | C4H6O4 | | 分子量: | 118.09 | | EINECS: | 203-740-4 | | カテゴリ情報: | Inhibitors;Heterocycles;alpha,omega-Alkanedicarboxylic Acids;alpha,omega-Bifunctional Alkanes;Monofunctional & alpha,omega-Bifunctional Alkanes;SuccinicSeries;Food & Feed ADDITIVES;ACS Grade;Building Blocks;C1 to C5;Carbonyl Compounds;Carboxylic Acids;Chemical Synthesis;Essential Chemicals;Inorganic Salts;Organic Building Blocks;Research Essentials;Solutions and Reagents;Fine chemicals;chemical reagent;pharmaceutical intermediate;phytochemical;reference standards from Chinese medicinal herbs (TCM).;standardized herbal extract;110-15-6;PBS | | Mol File: | 110-15-6.mol | 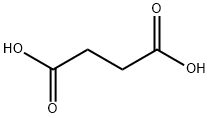 |
| 融点 | 185 °C | | 沸点 | 235 °C | | 比重(密度) | 1.19 g/mL at 25 °C(lit.) | | 蒸気圧 | 0-0Pa at 25℃ | | FEMA | 4719 | SUCCINIC ACID | | 屈折率 | n20/D 1.4002(lit.) | | 闪点 | >230 °F | | 貯蔵温度 | 2-8°C | | 溶解性 | Soluble in ethanol, ethyl ether, acetone and methanol. Insoluble in toluene, benzene, carbon disulfide, carbon tetrachloride and petroleum ether. | | 酸解離定数(Pka) | 4.16(at 25℃) | | 外見 | Powder/Solid | | 色 | White to off-white | | PH | 3.65(1 mM solution);3.12(10 mM solution);2.61(100 mM solution); | | 臭い (Odor) | at 100.00 %. wormwood | | においのタイプ | herbal | | 水溶解度 | 80 g/L (20 ºC) | | Merck | 14,8869 | | BRN | 1754069 | | Dielectric constant | 2.4(26℃) | | 安定性: | Stable. Substances to be avoided include strong bases, strong oxidizing agents. Combustible. | | InChIKey | KDYFGRWQOYBRFD-UHFFFAOYSA-N | | LogP | -0.59 | | CAS データベース | 110-15-6(CAS DataBase Reference) | | NISTの化学物質情報 | Butanedioic acid(110-15-6) | | EPAの化学物質情報 | Succinic acid (110-15-6) |
| 外観 | 白色, 結晶~結晶性粉末 | | 定義 | 本品は、次の化学式で表されるジカルボン酸である。 | | 溶解性 | 水, エタノールに可溶。熱水に極めて溶けやすく、水、エタノールにやや溶けやすく、ジエチルエーテルにやや溶けにくい。エタノール、メタノール、アセトン、熱水、熱ギ酸に可溶、エーテルに難溶。 | | 解説 | コハク酸脂肪族ジカルボン酸の一つで、ブタン二酸の別名をもつ。1550年にドイツのアグリコラがこはくを乾留して得たとの記録があることから、この名が与えられている。こはくにはコハク酸誘導体が含まれている。コハク酸は、天然には、二枚貝、地衣類、菌類などに含まれていて、広範囲の動植物に分布しており、貝類のうま味成分として知られている。フマル酸,マレイン酸の水素添加,リンゴ酸のヨウ化水素による還元などによって得られる.また,アルコール発酵でも得られる.無色の柱状あるいは板状結晶.融点185 ℃.1.572.エタノール,メタノール,アセトン,熱水などに可溶,エーテルに難溶.濃硫酸の存在下でアルコールと反応してジエステルが得られる.これはアンモニアと反応してスクシンアミドC2H4(CONH2)2を与える.コハク酸はその沸点235 ℃ まで加熱すると酸無水物である無水コハク酸を生じる.コハク酸のナトリウム塩は貝類のうま味成分であり,調味料に用いられる | | 用途 | 合成清酒、味噌、しょう油 | | 用途 | コハク酸およびそのナトリウム塩は食品衛生法により定められた指定食品添加物であり、食品の味をととのえる調味料や、食品に酸味を加える酸味料として用いられている。コハク酸がよく使われているのは、合成清酒、みそ、しょうゆなどの調味料で、合成酒中には0.08~0.09%使用されている。化粧品の成分としても用いられている例もある。 | | 構造 | コハク酸は、構造中にカルボン酸を2つ有するジカルボン酸化合物です。分子式C4H6O4、分子量118.09で表され、IUPAC命名法では、ブタン二酸という名称が与えられています。
コハク酸は、2つのカルボキシル基(-COOH)を持つ4炭素の直鎖状の分子です。
2つのカルボキシル基によって、酸性度が高く、水に溶けやすい性質を持ちます。また、カルボキシル基がプロトンを放出することにより、コハク酸は強い酸として振る舞います。
| | 化粧品の成分用途 | pH調整剤、香料 | | 説明 | Succinic acid is a dicarboxylic acid with a pair of carboxylic acid functional groups. The terminal carboxylic acid groups can react with primary amine groups in the presence of activators (e.g. EDC, or HATU) to form a stable amide bond. | | 化学的特性 | Succinic acid,C02H(CH2)2C02H, also known as butanedioic acid,butane diacid, and amber acid, is a colorless odorless prisms or white crystalline powder that melts at 185°C (364 of). Soluble in water and alcohol, it is used as a chemical intermediate, Succinic acid is used in lacquers,medicine,dyes,and as a taste modifier. | | 使用 | succinic acid is widely use as organic intermediates for the pharmaceutical, engineering plastics, resins etc.. For the synthesis of sedatives, contraceptives and cancer drugs in the pharmaceutical industry. In the chemical industry for the production of dyes, alkyd resin, glass fiber reinforced plastics, ion exchange resins and pesticides. | | 使用 | Succinic Acid was identified in essential oil from Saxifraga stolonifera and has antibacterial activity. | | 使用 | Succinic Acid is an acidulant that is commercially prepared by the
hydrogenation of maleic or fumaric acid. it is a nonhygroscopic acid
but is more soluble in 25°c water than fumaric and adipic acid. it
has low acid strength and slow taste build-up; it is not a substitute
for normal acidulants. it combines with proteins in modifying the
plasticity of bread dough. it functions as an acidulant and flavor
enhancer in relishes, beverages, and hot sausages. | | 定義 | A crystalline carboxylic
acid, HOOC(CH2)2COOH, that occurs in
amber and certain plants. It forms during
the fermentation of sugar (sucrose). | | 定義 | ChEBI: An alpha,omega-dicarboxylic acid resulting from the formal oxidation of each of the terminal methyl groups of butane to the corresponding carboxy group. It is an intermediate metabolite in the citric acid cycle. | | 生理学的効果 | 生体中においては、代謝過程における酸化還元反応で重要な役割を果たしていて、TCA回路の一員である。TCA回路においては、α(アルファ)-ケトグルタル酸の脱炭酸によってスクシニル補酵素A(活性コハク酸)が生成する。これはそのまま、ヘモグロビン、クロロフィル、チトクロムなどのポルフィリン環の合成に使われたり、ほかにエネルギーを与えて自らはコハク酸に分解したりする。コハク酸はコハク酸デヒドロゲナーゼ(脱水素酵素)により脱水素化されてフマル酸になり、チトクロム系に電子が伝達される。 | | Biotechnological Production | Traditionally, succinic acid is produced by petrochemical synthesis using the
precursor maleic acid. However, there are some microorganisms that are
able to produce succinic acid (e.g. Actinobacillus succinogenes, Anaerobiospirillum
succiniciproducens and Mannheimia succiniciproducens). Maximum product
concentrations of 106 g.L-1 with a yield of 1.25 mol of succinic acid per mole of
glucose and a productivity of 1.36 g.L-1.h-1 have been achieved by growing A.
succinogenes on glucose . A high productivity of 10.40 g.L-1.h-1 has been
reached with A. succinogenes growing on a complex medium with glucose in a
continuous process with an integrated membrane bioreactor-electrodialysis process.
In this process, the product concentration has been 83 g.L-1 . Moreover,
metabolic engineering methods were used to develop strains (e.g. C.
glutamicum, E. coli, S. cervisiae and Y. lipolytica) with high productivity and titer
as well as low byproduct formation. For example, growing C.
glutamicum strain DldhA-pCRA717 on a defined medium with glucose, a high
productivity of 11.80 g.L-1.h-1 with a yield of 1.37 mol of succinic acid per mole
of glucose and a titer of 83 g.L-1 has been reported after 7 h. An extended
cultivation resulted in a product concentration of 146 g.L-1 after 46 h. | | Synthesis Reference(s) | Canadian Journal of Chemistry, 56, p. 2269, 1978 DOI: 10.1139/v78-373
Synthesis, p. 709, 1984 DOI: 10.1055/s-1984-30945 | | 一般的な説明 | White crystals or shiny white odorless crystalline powder. pH of 0.1 molar solution: 2.7. Very acid taste. | | 空気と水の反応 | Slightly water soluble. | | 反応プロフィール | Succinic acid reacts exothermically to neutralize bases, both organic and inorganic. Can react with active metals to form gaseous hydrogen and a metal salt. Such reactions are slow in the dry, but systems may absorb water from the air to allow corrosion of iron, steel, and aluminum parts and containers. Reacts slowly with cyanide salts to generate gaseous hydrogen cyanide. Reacts with solutions of cyanides to cause the release of gaseous hydrogen cyanide. May generate flammable and/or toxic gases and heat with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. May react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Can be oxidized exothermically by strong oxidizing agents and reduced by strong reducing agents. May initiate polymerization reactions. | | 火災危険 | Flash point data for Succinic acid are not available. Succinic acid is probably combustible. | | 燃焼性と爆発性 | Non flammable | | 使用用途 | コハク酸は幅広い用途に用いられています。
食品業界では、うまみの添加剤や、香料、酸度調整剤などに使用されています。また、工業や医薬品製造においては、ポリエステルやポリウレタンといったポリマーや、樹脂、染料の製造、さまざまな医薬品を合成する際の中間体として使用されています。
1. 独特のうまみ味と食品添加物としての利用
コハク酸は、アサリなどの貝類に含まれるうま味成分として知られています。
2. 工業製品、医薬品としての利用
工業的には、さまざまな化合物の合成原料として利用されます。
3. 緩衝作用とpH調整剤としての利用
コハク酸は有機酸の一種であり、その共役塩基との混合物は緩衝作用を持つ事から、化粧品などのpH調整剤として用いられています。
| | Biochem/physiol Actions | Succinic acid is a byproduct of anaerobic fermentation in microbes. It is a dicarboxylic acid and an intermediate in Kreb′s cycle. Polymorphism in succinate dehydrogenase leads to succinate accumulation. High levels of succinate impairs 2-oxoglutarate epigenetic signalling. Succinate levels may modulate tumor progression. Succinate inhibits histone demethylation and may contribute to epigenetic changes. Succinate is crucial for interleukin-1 β (IL-1β) synthesis during inflammation and immune signalling. | | 生物学的応用 | Succinic acid and its derivatives are used as flavoring agents for food and beverages. This acid could be used as feedstock for dyes, insecticides, perfumes, lacquers, as well as in the manufacture of clothing, paint, links, and fibers (McKinlay et al. 2007). Succinic acid is widely used in medicine as an antistress, antihypoxic, and immunity-improving agent, in animal diets, and as a stimulator of plant growth. It is also a component of bio-based polymers such as nylons or polyesters (Kamzolova et al. 2012b). Succinate esters are precursors for the known petrochemical products such as 1,4-butanediol, tetrahydrofuran, c-butyrolactone, and various pyrrolidinone derivatives (Bechthold et al. 2008).
Succinic acid production by Y. lipolytica was reported for the first time when it was grown on ethanol under aerobic conditions and nitrogen limitation. Succinic acid amount was 63.4 g/L as the major product of batch fermentation in this process. However, the disadvantage was low yield of succinic acid on ethanol (58 %), and a high cost of production (Kamzolova et al. 2009).
Kamzolova et al. developed a novel process for the production of succinic acid. It includes the synthesis of a-ketoglutaric acid by a thiamine-auxotrophic strain Y. lipolytica VKMY-2412 from n-alkanes, and subsequent oxidation of the acid by hydrogen peroxide to succinic acid. The concentration of succinic acid and its yield were found to be 38.8 g/L and 82.45 % of n-alkane consumed, respectively (Kamzolova et al. 2012b).
Succinic acid production was also studied by genetically modified strains using glucose and glycerol as substrates. Yuzbashev et al. constructed temperaturesensitive mutant strains with mutations in the succinate dehydrogenase encoding gene SDH1 by in vitro mutagenesis-based approach. Then, the mutants were used to optimize the composition of the media for selection of transformants with the deletion in the SDH2 gene. The defects of each succinate dehydrogenase subunit prevented the growth on glucose, but the mutant strains grew on glycerol and produced succinate in the presence of the buffering agent CaCO3. Subsequent selection of the strain with deleted SDH2 gene for increased viability was allowed to obtain a strain that is capable to accumulate succinate at the level of more than 450 g/L with buffering and more than 17 g/L without buffering. Therefore, a reduced succinate dehydrogenase activity can lead to an increased succinate production (Yuzbashev et al. 2010). Y. lipolytica is able to produce succinic acid at low pH values. High amounts of succinate can be achieved by genetic engineering (Otto et al. 2013). | | 安全性プロファイル | Moderately toxic by subcutaneous route. A severe eye irritant. Mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes. | | 発がん性 | Monosodium succinate was
given to groups of 50 male and 50 female Fischer 344 rats
in drinking water at levels of 0%, 1%, or 2% for 2 years. No
toxic lesion specifically caused by long-term administration
of monosodium succinate was detected, and no dose-related
increase was found in the incidence of tumors in any organ or
tissue. The incidence of C-cell tumors of the thyroid gland of
females that received 2% solution was apparently, but not
significantly, higher than that in controls. Because C-cell
tumors are commonly occurring spontaneous tumors in aging
female rats of this strain and the incidence of C-cell tumors in
the female control group was lower than that of historical
controls for the testing laboratory, the authors concluded that
this lesion was not treatment related. | | 製造方法 | 工業生産の分野においては、もしくはその脱水環化物である無水マレイン酸を接触的に水素添加する事で調製します。
また、既述のようにコハク酸はTCA回路の中間体であることから、微生物を用いた発酵生産も可能です。ただし、その生産性は低くこれまで実用化には至っていません。しかし、(財)地球環境産 業技術研究機構(RITE)のグループは、グルタミン 酸生産菌である Corynebacterium glutamicumをメタノリックエンジニアリングの技術を用いて機能改変する事で、コハク酸の発酵生産効率を大幅に上げる事に既に成功しています。他にも、神戸大学のグループは、光合成微生物の一種であるラン藻の遺伝子組み換え体を用いてコハク酸の生産性を大きく向上される事に成功しています。このように、コハク酸の微生物発酵に関してはその開発が進みつつあるといえます。
- コハク酸が多く含まれる生物、食品
しじみ、あさり、かき、はまぐり
| | 純化方法 | Wash it with diethyl ether. Crystallise it from acetone, distilled water, or tert-butanol. Dry it under vacuum over P2O5 or conc H2SO4. Also purify it by conversion to the disodium salt which, after crystallisation from boiling water (charcoal), is treated with mineral acid to regenerate the succinic acid. The acid is then recrystallised and dried in a vacuum. [Beilstein 2 H 606, 2 IV 1908.] |
|