
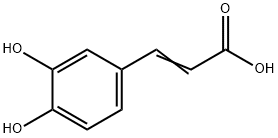
Caffeic acid
| Price | $1 |
| Package | 1KG |
| Min. Order: | 1G |
| Supply Ability: | 100KG |
| Update Time: | 2019-07-06 |
Product Details
| Product Name: Caffeic acid | CAS No.: 331-39-5 |
| Min. Order: 1G | Purity: 98% |
| Supply Ability: 100KG | Release date: 2019/07/06 |
AD68
| Product Name: | Caffeic acid |
| Synonyms: | RARECHEM BK HC T335;TIMTEC-BB SBB006475;(2E)-3-(3,4-Dihydroxyphenyl)-2-propenoic acid;2-Propenoic acid, 3-(3,4-dihydroxyphenyl)-;3-(3,4-dihydroxyphenyl)-2-propenoicaci;3-(3,4-Dihydroxyphenyl)propenoic acid;3,4-Dihydroxybenzeneacrylic acid;3,4-dihydroxybenzeneacrylicacid |
| CAS: | 331-39-5 |
| MF: | C9H8O4 |
| MW: | 180.16 |
| EINECS: | 206-361-2 |
| Product Categories: | Pharmaceutical Intermediates;Aromatic Cinnamic Acids, Esters and Derivatives;Cinnamic acid;Organic acids;Antioxidant;Biochemistry;Aromatics;sy;Berberis sp.;Bioactive Small Molecules for Epigenetic Research;DNA Methyltransferase Inhibitors;Epigenetics;Euphorbia sp;Allium cepa (Onion);Allium sativum (Garlic);Armoracia rusticana (Horseradish);Aspalathus linearis (Rooibos tea);Bioactive Small Molecules;Building Blocks;C9;Carbonyl Compounds;Carboxylic Acids;C-CH;Cell Biology;Chamaemelum nobile (Chamomile tea);Chemical Synthesis;Cichorium intybus (Chicory);Curcuma longa (Turmeric);Elettaria Cardamomum (Cardamom);Hordeum vulgare (Barley);Humulus lupulus (Hops);Hypericum perforatum (St John′;Linum usitatissimum (Flax);Molecular;s wort);API;chemical reagent;pharmaceutical intermediate;phytochemical;reference standards from Chinese medicinal herbs (TCM).;standardized herbal extract;Cosmetics;Inhibitors;Pyridines ,Halogenated Heterocycles ,Heterocyclic Acids;pharma. |
| Mol File: | 331-39-5.mol |
 |
|
| Caffeic acid Chemical Properties |
| Melting point | 211-213 °C (dec.)(lit.) |
| Boiling point | 272.96°C (rough estimate) |
| density | 1.2933 (rough estimate) |
| refractive index | 1.4500 (estimate) |
| storage temp. | Store at RT. |
| solubility | ethanol: 50 mg/mL |
| form | powder |
| color | yellow to tan |
| Water Solubility | soluble in hot water |
| Merck | 14,1635 |
| BRN | 2210883 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, strong bases. |
| CAS DataBase Reference | 331-39-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Cinnamic acid, 3,4-dihydroxy-(331-39-5) |
| EPA Substance Registry System | 2-Propenoic acid, 3-(3,4-dihydroxyphenyl)-(331-39-5) |
| Safety Information |
| Hazard Codes | Xn,Xi |
| Risk Statements | 36/37/38-40-63-68 |
| Safety Statements | 26-36/37/39-36 |
| WGK Germany | 3 |
| RTECS | GD8950000 |
| Hazard Note | Irritant |
| HS Code | 29182990 |
| Hazardous Substances Data | 331-39-5(Hazardous Substances Data) |
| Caffeic acid Usage And Synthesis |
| Physical and Chemical Properties | Caffeic acid, scientific name: "3-(3,4-dihydroxyphenyl)-2-propenoic acid." Molecular formula: C9H8O4; the molecular weight: 180.16. It is presented in plants such as coffee in the form of chlorogenic acid. It is yellow crystals with melting point being 223~225 °C. When it is precipitated in concentrated solution, it contains no crystal water. However, precipitate of crystals from dilute solution contains one molecule of crystallized water. It is slightly soluble in water, and easily soluble in hot water, cold ethanol, and ethyl acetate. Its alkaline solution is orange and exhibits dark green when being mixed with ferric chloride. Application: caffeic acid is safe to be applied in cosmetics and has a broader antibacterial and antiviral activity. It can also absorb ultraviolet radiation. A low concentration of it already has inhibitory efficacy on the generation of skin melanin. Its applied amount in the beauty products for whitening is at the range of 0.5 to 2%. It can also be used as additive for the oxidized hair dyes which is good for enhancing the strength of the color. Preparation: it can be produced from the Perkin’s reaction between protocatechuic aldehyde and acetic acid,As follows: 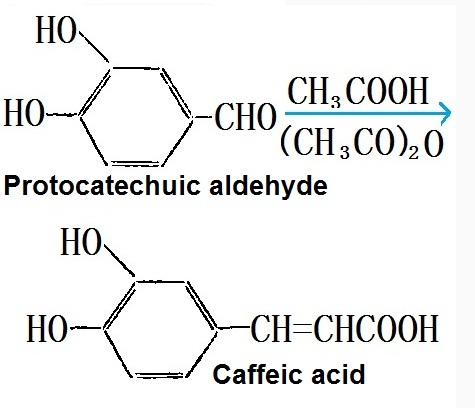 |
| Extraction Method | Caffeic acid belongs to common phenolic compounds with effects of increasing the levels of white blood cells. It is easily to be confused with caffeine and is widely distributed in the plant kingdom. Its major plant sources include lemon peel, Ranunculaceae cimicifuga rhizome, and valerian root. Caffeic acid, together with ferulic acid, erucic acid, and p-hydroxy cinnamic acid are ubiquitous hydroxy cinnamic acid-class molecule distributed in various kinds of plants. This kind of products has conjugated double bonds in the side chain of the molecular structure, and thus exhibiting significant fluorescence upon ultraviolet light, mostly showing bluish color fluorescence. This is a advantage for paper chromatography tests or thin layer chromatography tests. Extraction Method: Single spike cimicifuga rhizome is extracted with methanol which is removed through concentration under reduced pressure. Add hot water to the residue to dissolve it. Heating the water to dissolve the residue, and further filter the insolubles upon heating. After cooling, add benzene for extraction with the benzene solution being washed with 1% aqueous sodium bicarbonate and further collecting the washed solution. Add dilute hydrochloric acid for acidification, and then apply benzene to remove the free organic acid; Concentrate under reduced pressure to get rid of the benzene with the residue being the enriched product of caffeic acid. The above information is edited by the chemicalbook of Dai Xiongfeng. |
| Drugs for white blood cells increase | Caffeic acid is a kind of drugs for stopping bleeding as well as increasing the number of white blood cell with effects of contracting microvascular coagulation, improving the function of coagulation factors, and increasing white blood cells and platelets. It is suitable for preventing bleeding or stopping bleeding in surgeries as well as for stopping bleeding for bleeding diseases in internal medicine, and obstetrics and gynecology. It is also suitable for treating leukopenia and thrombocytopenia caused by a variety of reasons. |
| HPLC determination of caffeic acid in dandelion | [For the test] Compositae dandelion: Taraxacum mongolicum Hand. Mazz, Alkali land dandelion T. sinicum Kitag. (1) Chromatographic conditions: take octadecylsilane silica gel as a filling agent; methanol-phosphate buffer (sodium dihydrogen phosphate 1.56g, add water to dissolve it in 1000 ml, add 1% of phosphoric acid solution for adjusting to pH 3.8 to 4.0, that’s it) (23:77) as the mobile phase; detection wavelength is 323 nm; column temperature should be 40 °C. Number of theoretical plates should be calculated according to the caffeic acid peak and should not be less than 3,000. (2) the preparation of the reference solution: take 7.5 mg of caffeic acid reference substance, accurately weigh it and transfer it into the 50ml volumetric flask; add methanol to certain scale, shake; take precise amount of 2ml and put into 10ml volumetric flask, add methanol to the scale , shake, to obtain the reference solution (containing caffeic acid 30μg per ml). (3) Preparation of sample solution: Take about 1 g of medicine powder, accurately weigh it, and put it in 50ml Erlenmeyer flask, precisely add 10 mL of methanol solution containing 5% formic acid, seal, shake, weigh, and subject to ultrasonic treatment for 30min, remove, cool, then weigh again; use the methanol solution of 5% formic acid to complement the weight loss, shake, centrifuge, and take the supernatant for being filtrated through microporous membrane (0.45μm) with the filtrate being placed in brown bottle to obtain the sample solution. (4) Determination: separately and precisely pipette 10μl of both reference solution and sample solution and transfer into the liquid chromatography for measurement. (5) Measurement results: calculated from the dry products of dandelion herbs, the caffeic acid content should not be less than 0.02%. |
| Chemical Properties | It is yellow crystals and can be dissolved in water and ethanol. |
| Uses | 1. Reagents for Organic Synthesis. 2. Intermediate of caffeic acid; can be used in organic synthesis. 3. Used for Biochemical studies. |
| Category | Toxic Chemicals |
| Toxicity grading | Poisoning |
| Acute toxicity | Intraperitoneal administration-rat LDL0: 1500 mg/kg |
| Flammability and hazard characteristics | Thermal decomposition causes irritating smoke |
| Storage Characteristics | Treasury: ventilation low-temperature and dry. |
| Chemical Properties | Light yellow to greenish-yellow powder |
| Uses | antineoplastic, PGE2 synthase inhibitor, PK inhibitor |
| Uses | Caffeic Acid is a constituent of plants, probably occurs in plants only in conjugated forms. Caffeic acid is found in all plants because it is a key intermediate in the biosynthesis of lignin, one of the principal sources of biomass. Caffeic acid is one of the main natural phenols in argan oi. |
| Extinguishing agent | Water, powder, CO2, foam. |
| General Description | Yellow prisms or plates (from chloroform or ligroin) or pale yellow granules. Alkaline solutions turn from yellow to orange. |
| Air & Water Reactions | Insoluble in water. |
| Reactivity Profile | Caffeic acid is a carboxylic acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in Caffeic acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions. |
| Health Hazard | ACUTE/CHRONIC HAZARDS: When heated to decomposition Caffeic acid emits acrid smoke and fumes. |
| Fire Hazard | Flash point data for Caffeic acid are not available; however, Caffeic acid is probably combustible. |
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $29.00/100mg |
VIP6Y
|
TargetMol Chemicals Inc.
|
2026-02-24 | |
| $0.00/25kg |
VIP2Y
|
Shaanxi Dideu New Materials Co. Ltd
|
2026-01-04 | |
| $0.00/25kg |
VIP4Y
|
Nanjing Shunxiang Pharmaceutical Technology Co.,Ltd.
|
2025-09-28 | |
| $0.00/1kg |
VIP2Y
|
Shaanxi Xianhe Biotech Co., Ltd
|
2025-04-27 | |
| $10.00/1KG |
VIP7Y
|
Hebei Chuanghai Biotechnology Co., Ltd
|
2024-11-29 | |
| $0.00/1kg |
VIP2Y
|
Watson Biotechnology Co.,Ltd
|
2024-10-10 | |
| $5.00/1KG |
VIP5Y
|
Hebei Chuanghai Biotechnology Co,.LTD
|
2024-08-21 | |
| $6.00/1kg |
HebeiShuoshengImportandExportco.,Ltd
|
2024-08-07 | ||
| $0.00/1kg |
VIP2Y
|
JINING XINHE CHEMICAL CO., LTD
|
2024-07-20 | |
| $6.00/1kg |
Hebei Longbang Technology Co., Ltd
|
2024-05-08 |
- Since: 2014-12-17
- Address: Zhengzhou High tech Zone, Henan Province, China
INQUIRY
杨俊青
sales@coreychem.com
sales@coreychem.com




![1-(6-Chlorophenyl-2,3,4,5-d4)-7-oxabicyclo[4.1.0]heptane](https://img.chemicalbook.com/ProductImageEN/2018-8/Large/db5b5bbd-5d2b-46ce-9032-e7f884bb46ad.jpg)


 China
China