
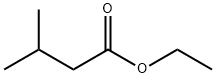
Ethyl isovalerate
| Price | $1 |
| Package | 1kg |
| Min. Order: | 1 g |
| Supply Ability: | 100KG |
| Update Time: | 2019-07-06 |
Product Details
| Product Name: Ethyl isovalerate | CAS No.: 108-64-5 |
| EC-No.: 203-602-3 | Min. Order: 1 g |
| Purity: 99% | Supply Ability: 100KG |
| Release date: 2019/07/06 |
AD68
| Ethyl isovalerate Basic information |
| Description References |
| Product Name: | Ethyl isovalerate |
| Synonyms: | ISOVALERIC ACID ETHYL ESTER;ISOVALERIC ACID:ETHYL ESTER;FEMA 2463;ETHYL ISOVALERATE;ETHYL ISOPENTANOATE;ETHYL BETA-METHYLBUTYRATE;ETHYL 3-METHYLBUTANOATE;3-METHYL BUTYRIC ETHYL ESTER |
| CAS: | 108-64-5 |
| MF: | C7H14O2 |
| MW: | 130.18 |
| EINECS: | 203-602-3 |
| Product Categories: | Organics;Alphabetical Listings;E-F;Flavors and Fragrances;Certified Natural ProductsFlavors and Fragrances;C6 to C7;Carbonyl Compounds;Esters;Alpha Sort;Chemical Class;E;E-LAlphabetic;EQ - EZAnalytical Standards;Volatiles/ Semivolatiles;Building Blocks;C6 to C7;Carbonyl Compounds;Chemical Synthesis;Organic Building Blocks |
| Mol File: | 108-64-5.mol |
 |
|
| Ethyl isovalerate Chemical Properties |
| Melting point | -99 °C |
| Boiling point | 131-133 °C(lit.) |
| density | 0.864 g/mL at 25 °C(lit.) |
| vapor pressure | 7.5 mm Hg ( 20 °C) |
| refractive index | n20/D 1.396(lit.) |
| FEMA | 2463 | ETHYL ISOVALERATE |
| Fp | 80 °F |
| storage temp. | Flammables area |
| solubility | 2.00g/l |
| form | Liquid |
| color | Clear colorless to pale yellow |
| Merck | 14,3816 |
| BRN | 1744677 |
| CAS DataBase Reference | 108-64-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Butanoic acid, 3-methyl-, ethyl ester(108-64-5) |
| EPA Substance Registry System | Butanoic acid, 3-methyl-, ethyl ester(108-64-5) |
| Safety Information |
| Risk Statements | 10 |
| Safety Statements | 16 |
| RIDADR | UN 3272 3/PG 3 |
| WGK Germany | 2 |
| RTECS | NY1504000 |
| F | 13 |
| TSCA | Yes |
| HazardClass | 3 |
| PackingGroup | III |
| MSDS Information |
| Provider | Language |
|---|---|
| Ethyl isovalerate | English |
| SigmaAldrich | English |
| ACROS | English |
| ALFA | English |
| Ethyl isovalerate Usage And Synthesis |
| Description | Ethyl isovalerate is the ethyl ester form of isovalerate formed between ethyl alcohol with isovaleric acid. It is a derivative of valeric acid, mainly found in fruits (one of the major component of blueberry). It is a kind of natural food flavoring agent with a fruity type odor and flavor. It is widely used in perfumery and fragrance. It is now frequently synthesized using surfactant-coated lipase (various kinds of origins) immobilized in magnetic nanoparticles. |
| References | Luebke, William. "ethyl isovalerate108-64-5." (2015). And, Hiannie Djojoputro, and S. Ismadji. "Density and Viscosity Correlation for Several Common Fragrance and Flavor Esters." Journal of Chemical & Engineering Data 50.2(2005):págs. 727-731. Mahmood, Iram, et al. "A surfactant-coated lipase immobilized in magnetic nanoparticles for multicycle ethyl isovalerate enzymatic production." Biochemical Engineering Journal 73.8(2013):72-79. |
| Chemical Properties | clear colorless to pale yellowish liquid |
| Definition | ChEBI: The fatty acid ethyl ester of isovaleric acid. |
| Uses | In alcohol solution for flavoring confectionery and beverages. |
| General Description | A colorless oily liquid with a strong odor similar to apples. Less dense than water. Vapors heavier than air. Flash point 77°F. May mildly irritate skin and eyes. |
| Air & Water Reactions | Highly flammable. Slightly soluble in water. |
| Reactivity Profile | ETHYL ISOVALERATE is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. |
| Health Hazard | Inhalation or contact with material may irritate or burn skin and eyes. Fire may produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution. |
| Purification Methods | Wash the ester with aqueous 5% Na2CO3, then saturated aqueous CaCl2. Dry it over CaSO4 and distil. [Beilstein 2 IV 898.] |
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $10.00/1KG |
VIP5Y
|
Hebei Chuanghai Biotechnology Co,.LTD
|
2024-08-22 | |
| $0.00/25kg |
VIP2Y
|
Shaanxi Dideu New Materials Co. Ltd
|
2025-12-22 | |
| $0.00/1Kg |
VIP1Y
|
Chongqing Soarwin Technology Co., Ltd
|
2025-04-14 | |
| $0.00/1KG |
VIP7Y
|
Hebei Chuanghai Biotechnology Co., Ltd
|
2025-03-14 |
- Since: 2014-12-17
- Address: Zhengzhou High tech Zone, Henan Province, China
INQUIRY
杨俊青
sales@coreychem.com
sales@coreychem.com





 China
China