
Product Details
| Product Name: BISMUTH SUBSALICYLATE | CAS No.: 14882-18-9 |
| EC-No.: 238-953-1 | Min. Order: 1KG |
| Purity: 99% | Supply Ability: 50000KG/month |
| Release date: 2025/08/08 |
| CAS: | 14882-18-9 |
| MF: | C7H5BiO4 |
| MW: | 362.09 |
| EINECS: | 238-953-1 |
| Product Categories: | Organometallics;HALINONE |
| Mol File: | 14882-18-9.mol |
 | |
| BISMUTH SUBSALICYLATE Chemical Properties |
| Melting point | >350 °C(lit.) |
| density | 3.05[at 20℃] |
| vapor pressure | 0Pa at 20℃ |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Practically insoluble in water and in alcohol. It dissolves in mineral acids with decomposition. |
| form | Powder |
| color | White |
| Odor | odorless |
| Water Solubility | Practically insoluble in water, alcoholSoluble in acid and alkali. Insoluble in cold water, ethanol, diethyl ether and n-octanol. |
| Merck | 14,1285 |
| BRN | 9170211 |
| InChIKey | ZREIPSZUJIFJNP-UHFFFAOYSA-K |
| LogP | 0.11 at 25℃ |
| EPA Substance Registry System | Bismuth subsalicylate (14882-18-9) |
| BISMUTH SUBSALICYLATE Usage And Synthesis |
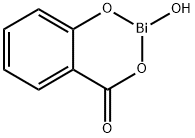
| Overview | Bismuth subsalicylate, an active ingredient in the popular medication Pepto-Bismol, is used for the treatment of nausea, heartburn, indigestion, upset stomach, diarrhea, and other temporary discomforts of the stomach and gastrointestinal tract (including duodenal and peptic ulcers, ulcerative colitis, and diarrhea[1–5]. It is also the main ingredient of Kaopectate. It displays anti-inflammatory action (due to salicylic acid) and also can also act as an antacid and mild antibiotic. In conjunction with antibiotic compounds such as tetracycline, clarithromyacin, and amoxicillin, bismuth subsalicylate has been effective in the treatment and eradication of Helicobacter pylori, the bacterium linked to gastritis and ulceration implicated in the development of gastric cancer and gastric lymphoma[6–9]. Bismuth subsalicylate has the empirical chemical formula of C7H5BiO4[10], and it is a colloidal substance obtained by hydrolysis of bismuth salicylate (Bi(C6H4(OH)CO2)3). Bismuth subsalicylate is one of the most widely used medicines in this country, estimated to be present in 60% of American households[11]. It has become the medication of choice for the prevention of traveler’s diarrhea[12], is useful for other forms of toxigenic diarrhea[13] and has been a commonly prescribed antidiarrheal medication for non-syndromic episodic diarrhea in both children and adults for nearly a century[14–17]. Although its detailed mode of action is still unknown, it may stimulate fluid and electrolyte absorption and adsorbs bile acids and enterotoxins[18]. In addition to this antidiarrheal activity, bismuth subsalicylate has well-known antibacterial properties[19, 20], Furthermore, because of its salicylate moiety and perhaps because of bismuth as well, bismuth subsalicylate is thought to be anti-inflammatory[13]. BSS is an old drug and as early as in 1857 Dr Chambers reported on its efficacy in the management of several diseases of the digestive tract[21]. It is commonly available in the USA and in several other countries without the need of a prescription and is largely used for a wide pattern of gastrointestinal diseases. 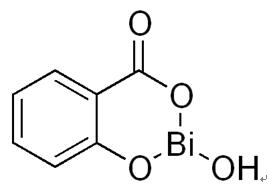 Figure 1. The chemical formula of the bismuth subsalicylate |
| Bismush salts | Bismuth salts have been promoted for therapeutic use for over two centuries for a range of clinical conditions including dyspepsia, diarrhoea, syphilis, oral and upper respiratory tract infections, veruccae and warts[22-25]. The bismuth salts or complexes used have also been diverse including such compounds as bismuth subnitrate, bismuth subgallate, bismuth subsalicylate, and bismuth subcarbonate as well as tripotassium dicitrato bismuthate. The use of the majority of bismuth salts has fallen into dis-favour because of the potential adverse effects, and indeed a number of drug-regulatory authorities are actively delisting bismuth-containing products from medicinal use. Nonetheless, over recent years two bismuth compounds, bismuth subsalicylate and bismuth sub-citrate, have attracted renewed interest because of their newly discovered activities for conditions which respond sub-optimally to existing therapies: travellers’ diarrhoea and peptic ulcer disease, respectively. In travellers’ diarrhoea, effective prophylaxis has been claimed for bismuth subsalicylate[26-29] while in peptic ulcer disease, relapse rates have been shown to be lower following bismuth sub-citrate therapy than following histamine H2antagonist therapy[30-32]. |
| Applications | Bismuth subsalicylate is used for the treatment of diarrhea, heartburn, colitis, upset stomach and other temporary discomforts of the stomach and gastrointestinal tract in adults and children 12 years of age and older[5, 33]. Bismuth subsalicylate belongs to a class of medications called antidiarrheal agents. It works by decreasing the flow of fluids and electrolytes into the bowel, reduces inflammation within the intestine, and may kill the organisms that can cause diarrhea. It shows certain antibacterial activity[19, 20]. |
| Pharmacokinetics | Bismuth subsalicylate displays anti-inflammatory action[due to salicylic acid] and can also act as an antacid and mild antibiotic. It can cause a black tongue and black stools in some users of the drug, when it combines with trace amounts of sulfur in their saliva and gastrointestinal tract. This discoloration is temporary and harmless[5]. In vitro dissociation data and in vivo animal data has demonstrated that bismuth subsalicylate is largely hydrolyzed in the stomach to bismuth oxychloride and salicylic acid. When reaching the small intestine, nondissociated bismuth subsalicylate can further react with other anions[bicarbonate and phosphate] to form insoluble bismuth salts. In the colon, nondissociated bismuth subsalicylate and other bismuth salts react with hydrogen sulfide to produce bismuth sulfide, a highly insoluble black salt responsible for the darkening of the stools[5]. |
| Mode of action | As an antidiarrheal, the exact mechanism still remains unclear. Several in vitro studies have shown that the mechanisms of bismuth subsalicylate are related to two distinct effects: an action directed against pathogenic microorganisms[34] and an intestinal antisecretory or proabsorptive effect on ion and water transport. This is clearly demonstrated for aspirin[which is a salycilate compound] and probably related to the inhibition of prostaglandin synthesis[35]. Experimental data are indeed solid and consistent and provide the basis to support the concept that bismuth subsalicylate is an anti-diarrhoea drug. Bismuth subsalicylate may exert its antidiarrheal action not only by stimulating absorption of fluid and electrolytes across the intestinal wall[antisecretory action] but also, when hydrolyzed to salicylic acid, by inhibiting synthesis of a prostaglandin responsible for intestinal inflammation and hypermotility. In addition, bismuth subsalicylate binds toxins produced by Escherichia coli. Both bismuth subsalicylate and the intestinal reaction products, bismuth oxychloride and bismuth hydroxide, are believed to have bactericidal action. As an antacid, bismuth has weak antacid properties[5]. |
| Side effects | Side effect associated with the bismuth subsalicylate include anxiety, any loss of hearing, confusion, constipation, diarrhea, difficulty in speaking or slurred speech, dizziness or lightheadedness, drowsiness, fast or deep breathing, headache[severe or continuing], increasing sweating, increased thirst, mental depression, muscle spasms, muscle weakness, nausea or vomiting, ringing or buzzing in ears, stomach pain, trembling, uncontrollable flapping movements of the hands[especially in elderly patients] or other uncontrolled body movements vision problems[36]. |
| Precautions | Before taking bismuth subsalicylate, you should consult your doctor in the following few conditions as detailed below[33]:
|
| References |
|
| Chemical Properties | White or almost white powder. |
| Uses | anticoagulant |
| Uses | Used to treat nausea, heartburn, indigestion, upset stomach, diarrhea, and other temporary discomforts of the stomach and gastrointestinal tract. |
| Uses | To impart pearly surface to cellulose-base, polystyrene, and phenolformaldehyde resins. Typical tap bulk density 0.43g/cm^3Bismuth subsalicylate is used to treat temporary discomforts of the stomach and gastrointestinal tract, such as diarrhea, indigestion, heartburn and nausea. It also acts as an antacid. It is practiced to decrease the number of bowel movements and to make the stool firmer. It is also used to reduce inflammation within the intestine. It is further used in growth media for selective isolation of microorganisms. |
| Definition | ChEBI: Bismuth subsalicylate is a bismuth salt of salicylic acid. It is a member of salicylates and a bismuth coordination entity. |
| Indications | Bismuth subsalicylate (Pepto-Bismol) also binds intestinal toxins and may coat irritated mucosal surfaces. |
Product picture

Packing &shipping&Payment
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $89.00/10g |
VIP4Y
|
TargetMol Chemicals Inc.
|
2025-10-27 | |
| $89.00/10g |
VIP6Y
|
TargetMol Chemicals Inc.
|
2025-10-27 | |
| $0.00/15kg |
VIP5Y
|
WUHAN FORTUNA CHEMICAL CO., LTD
|
2025-07-10 | |
| $89.00/10g |
VIP2Y
|
TargetMol Chemicals Inc.
|
2025-04-30 | |
| $60.00/1kg |
Hebei Zhuanglai Chemical Trading Co.,Ltd
|
2024-05-23 | ||
| $0.00/1Kg |
airuikechemical co., ltd.
|
2024-04-09 | ||
| $0.00/1Kg |
Changzhou Rokechem Technology Co., Ltd.
|
2024-01-29 | ||
| $100.00/1kg |
Henan Bao Enluo International TradeCo.,LTD
|
2023-07-31 | ||
| $1.10/1g |
VIP5Y
|
Dideu Industries Group Limited
|
2021-07-15 | |
| $15.00/1KG |
Zhuozhou Wenxi import and Export Co., Ltd
|
2021-07-09 |
+86-13288715578
sales@sichuanzyyt.com











 China
China