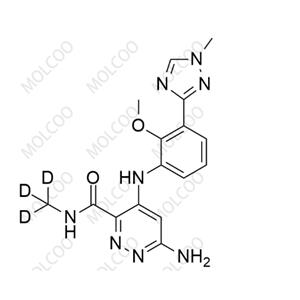
Deucravacitinib Impurity5;1609394-23-1
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Product Code:D084005
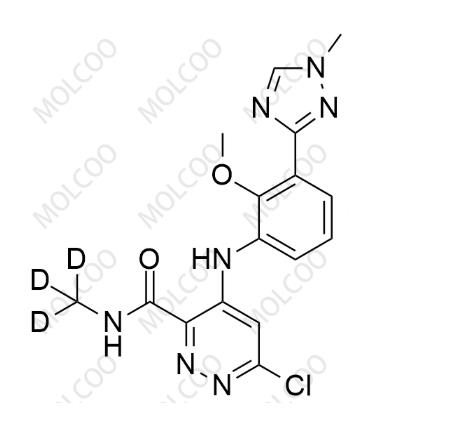
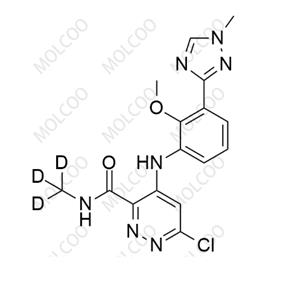
English Name:Deucravacitinib Impurity 5
English Alias:6-chloro-4-((2-methoxy-3-(1-methyl-1H-1,2,4-triazol-3-yl)phenyl)amino)-N-(methyl-d3)pyridazine-3-carboxamide
CAS No.:1609394-23-1
Molecular Formula:C₁₆H₁₃D₃ClN₇O₂
Molecular Weight:376.82
High-Purity Isotopic Standard:Confirmed by HPLC (≥99.0%), combined with NMR (including proton spectrum, carbon spectrum and deuterium signal analysis), HRMS (high-resolution mass spectrometry for precise confirmation of isotopic labeling) and elemental analysis, the structure is comprehensively verified. The labeling rate of the deuterated site (methyl-d3) is ≥99.5%, providing an accurate isotopic internal standard for Deucravacitinib impurity analysis.
Excellent Stability:Stable for 36 months under -20℃ light-protected and sealed storage conditions; the degradation rate is less than 0.1% within 6 months in methanol - water (1:1) mixture, and the deuterium labeling is firm without detachment risk, meeting the needs of long-term complex experiments and quality control.
Precise Quantitative Analysis:As a deuterated internal standard for UPLC-MS/MS detection, it effectively corrects matrix effects, significantly improves the quantitative accuracy of homologous impurities in Deucravacitinib API and formulations, with the relative standard deviation (RSD) of quantitative results <2%, meeting the stringent requirements of various pharmacopoeias for impurity quantification.
Metabolic Mechanism Research:With the help of deuterium isotope tracing technology, deeply analyze the metabolic pathways of impurities in vivo, such as amino acetylation, chlorine atom substitution reaction, methoxy demethylation process, etc., providing key data for optimizing the synthesis process of Deucravacitinib and evaluating the toxicological risks of impurities.
In-Depth Method Validation:When developing impurity detection methods, it is used to verify the resolution of UPLC (ensuring ≥2.8), the linear range of isotopic response of mass spectrometry (0.005 - 10 ng/mL), and precisely control the limit of detection (LOD) at 0.003 ng/mL, ensuring the scientificity and reliability of the detection method.
Deucravacitinib is a selective tyrosine kinase 2 (TYK2) inhibitor used for the treatment of autoimmune diseases such as psoriasis, exerting therapeutic effects by precisely regulating intracellular signaling pathways. Impurity 5, a deuterated process impurity introduced during its synthesis, may arise from side reactions in the chlorination of the pyridazine ring, methylation of the triazole ring, or N-methyl deuteration steps. The chlorine atom, deuterated methyl group, triazole ring and pyridazine carboxamide group in the structure of this impurity are similar to those of the main drug, enabling it to effectively eliminate matrix interference during biological sample detection. In the process of drug research and development and production, strict control of impurities is related to drug safety and effectiveness. Especially, deuterated impurities can reduce isotopic effects and improve quantification accuracy in pharmacokinetic studies. Therefore, the research on Impurity 5 is of great significance for the quality control of Deucravacitinib.
Advanced Detection Technology:Using UPLC-MS/MS technology, combined with a C18 column (1.7μm) and 0.1% formic acid - acetonitrile gradient elution, impurities can be efficiently separated within 7 minutes. The deuterated isotope peak (m/z difference of 3 Da) is baseline separated from non-deuterated impurities, with a detection limit as low as 0.001 ng/mL, enabling highly sensitive detection of trace impurities.
Clear Formation Mechanism:This impurity is formed by the chlorination reaction of 4-((2-methoxy-3-(1-methyl-1H-1,2,4-triazol-3-yl)phenyl)amino)pyridazine-3-carboxamide, followed by N-methyl deuteration reaction with deuterated methylamine (ND₃·DCl) under the action of a condensing agent. Studies have shown that optimizing the chlorination reaction temperature (controlled at 10 - 20℃), precisely adjusting the dosage of deuterated reagent and reaction time can significantly reduce the generation of by-products.
Comprehensive Safety Evaluation:In vitro cytotoxicity test data shows that the IC₅₀ of this impurity against HaCaT cells is 198.7 μM (Deucravacitinib IC₅₀ = 7.5 μM), with significantly lower toxicity than the main drug, but its content in drugs still needs to be strictly controlled. Currently, long-term stability tests (set at 60℃/90%RH) are being carried out to systematically monitor potential risks such as chlorine atom substitution and amide bond hydrolysis of this impurity under extreme conditions
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!









 China
China