
Product Details
| Product Name: Docetaxel | CAS No.: 114977-28-5 |
| EC-No.: 601-339-2 | Min. Order: 1kg |
| Purity: 99% | Supply Ability: 2000ton |
| Release date: 2024/04/12 | |
| Appearance: White Powder |
Docetaxel is a taxoid drug that inhibits the mitosis and proliferation of cancer cells by promoting the assembly of microtubule dimers into microtubules, while stabilizing the microtubules by preventing the depolymerization process, and blocking cells in G2 and M stages. Docetaxel has stronger pharmacological action than paclitaxel, its intracellular concentration is 3 times higher than that of paclitaxel, and its intracellular retention time is longer, and its affinity to microtubules is 2 times that of paclitaxel. As a microtubule stabilizer and assembly accelerator, the activity is 2 times greater than that of paclitaxel. As a microtubule depolymerization inhibitor, it is 2 times more active than paclitaxel. In vitro antitumor activity tests, it has been confirmed that docetaxel's antitumor activity is 1.3 to 12 times that of paclitaxel. Clinical studies have shown that docetaxel is more effective than paclitaxel for anthracycline-resistant breast cancer. Docetaxel is by far the most effective second-line treatment for anthracycline-resistant breast cancer; Docetaxel is one of the most effective agents in both monotherapy and combination chemotherapy for non-small cell lung cancer. Genotoxicity: Docetaxel showed cleavage in the chromosomal aberration test of CHO-K1 cells and mouse bone marrow micronucleus test, but no mutagenicity in Ames test and CHO/HGPRT gene mutation test.
Reproductive toxicity: Intravenous administration of docetaxel 0.3mg/kg in rats (about 1/50 of the clinically recommended dose based on body surface area) did not affect fertility, but caused testicular weight loss. The results were correlated with 10 cycles of repeated dosing in rats and dogs (once every 21 days for 6 months). Testicular atrophy and degeneration were observed at intravenous doses of 5mg/kg in rats and 0.375mg/kg in dogs (about 1/3 and 1/15 of the clinically recommended dose in terms of body surface area, respectively), and similar effects were observed in rats with increased dosing times at low doses. Use of docetaxel during pregnancy can cause fetal injury. Rats and rabbits given docetacel ≥0.3mg/kg/ day and 0.03mg/kg/ day (equivalent to 1/50 and 1/300 of the recommended clinical daily dose in terms of body surface area, respectively) during organ formation showed fetal and fetal toxicity (manifested by intrauterine death, increased absorption, fetal weight loss, and delayed ossification). These doses can also cause maternal toxicity. There is currently no sufficient and tightly controlled clinical data on pregnant women. If the patient uses this product during pregnancy, or becomes pregnant while using this product, she should be informed of the potential harm to the fetus and the potential risk of miscarriage. Women at risk of childbearing should avoid pregnancy during treatment with this product. It is not known whether docetaxel is excreted from human milk. Given that many drugs can be excreted from human milk and that docetaxel can cause serious adverse reactions in nursing infants, mothers should stop breastfeeding before using this product.
indication
1. Suitable for the treatment of locally advanced or metastatic breast cancer.
2. It is suitable for the treatment of locally advanced or metastatic non-small cell lung cancer, even after the failure of cisplatin based chemotherapy.
Side effects and adverse reactions
1. Myelosuppression: Neutropenia is the most common adverse event and is usually severe (less than 500 cells /mm3). Reversible and non-cumulative. Fever and infection associated with neutropenia have been reported in the literature. Anemia is seen in most cases, and severe thrombocytopenia occurs in a few cases.
2. Anaphylaxis: Severe anaphylaxis may occur in some cases, characterized by hypotension and bronchospasm, requiring interruption of treatment. Patients return to normal after discontinuation of the infusion and immediate treatment. Mild allergic reactions may also occur in some cases. Such as flushing, itchy red spots with or without use, chest tightness, back pain, difficulty breathing, drug fever or chills.
3. The skin reaction is often characterized by erythema, mainly seen on the hands and feet, but also local rashes on the arms, face and chest, sometimes accompanied by itching. The rash may usually occur within a week after a docestat infusion, but may recover before the next infusion. Severe symptoms such as peeling after a rash are rare. Finger (toe) nail lesions may occur, characterized by pigmentation or lightening, and sometimes pain and nail loss.
4. Fluid retention includes edema, and rare cases of pleural effusion, ascites, pericardial effusion, increased capillary permeability and weight gain have also been reported. After 4 cycles of treatment or a cumulative dose of 400mg/m2, fluid retention occurs in the lower limbs and may progress to systemic edema, accompanied by weight gain of 3 kg or more. After stopping docetaxel treatment, fluid retention gradually disappeared. To reduce fluid retention, patients should be given prophylactic corticosteroids.
5. Gastrointestinal reactions such as nausea, vomiting or diarrhea may occur.
6. Neurotoxicity has been reported in clinical trials.
7. Cardiovascular adverse reactions such as hypotension, sinus tachycardia, palpitation, pulmonary edema and hypertension may occur.
8. Other adverse reactions include: alopecia, weakness, mucositis, arthralgia and myalgia, hypotension, and injection site reactions.
9. Elevated aminotransferase and elevated bilirubin were also observed in patients with normal liver function during treatment, and the relationship between them and docetaxel remains unclear.
Matters needing attention
1. Patients with a history of severe allergy to docetaxel or Tween-80; 2. Patients with leukocyte count less than 1500/mm3; 3. Patients with severe damage to liver function. [Medication for pregnant and lactating women] There is no sufficient and strictly controlled clinical data on pregnant women. If the patient uses this product during pregnancy, or becomes pregnant while using this product, she should be informed of the potential harm to the fetus and the potential risk of miscarriage. Women at risk of childbearing should avoid pregnancy during treatment with this product. It is not known whether docetaxel is excreted from human milk. Given that many drugs can be excreted from human milk and that docetaxel can cause serious adverse reactions in nursing infants, mothers should stop breastfeeding before using this product. The efficacy and safety of docetaxel in children have not been determined. [Drug interactions] In vitro studies have shown that CYP3A4 inhibitors may interfere with the metabolism of this product, so caution should be exercised when used together with such drugs (such as ketoconazole, erythromycin, cyclosporine, etc.). In the event of an overdose, the patient should be moved to a special care unit and vital organ function should be closely monitored. When docetaxel is overdosed, there is no antidote. The main complications of predictable excess include neutropenia, skin reactions, and paresthesia.
Application:
1 Antitumor botanic drug for the treatment of metastatic breast cancer and non-small cell lung cancer.
2 Docetaxel can be used to study antibiotics, cell biology, cell signaling, neuroscience, apoptosis, and the cell cycle. Docetaxel is also used to study hair loss caused by docetaxel chemotherapy, prevention, and treatment of non-small cell lung cancer in patients contraindicated with standard chemotherapy. Docetaxel has also been used to study the effects of hypoxia-inducible factors-1α and 2α in androgen-insensitive prostate cancer cells.
3 A taxoid anticancer drug that binds and stabilizes the tubulin subunit, mitotic spindle depolymerization, resulting in cell cycle arrest and apoptosis
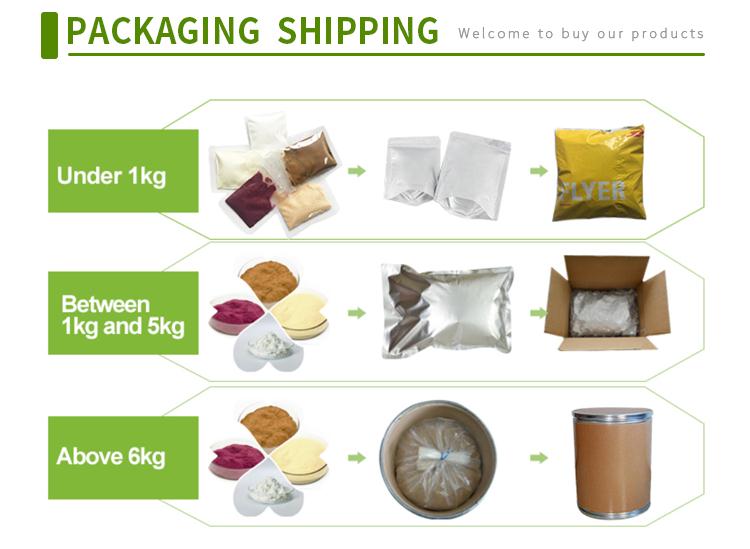
Storage Condition | Keep in a cool and dry place |
Transportation | By Sea or by Air(DHL/UPS/TNT/FEDEX/EMS) |
Delivery Time | 7-28 days |
Payment | T/T, Western Union or Bitcoin |









Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $30.00/25mg |
VIP2Y
|
TargetMol Chemicals Inc.
|
2025-12-11 | |
| $30.00/25mg |
VIP6Y
|
TargetMol Chemicals Inc.
|
2025-12-11 | |
| $2.00/100kg |
VIP1Y
|
ZHENGZHOU JIUYI TIME NEW MATERIALS CO,.LTD
|
2025-08-08 | |
| $0.00/1kg |
VIP2Y
|
Fuzhou Lista Biotechnology Co.,Ltd
|
2025-06-13 | |
| $5.00/1KG |
VIP1Y
|
Wuhan JiyunZen Tech Co., Ltd.
|
2025-06-06 | |
| $/ |
VIP5Y
|
RongNa Biotechnology Co.,Ltd
|
2025-04-29 | |
| $30.00/25mg |
VIP4Y
|
TargetMol Chemicals Inc.
|
2025-04-29 | |
| $0.00/1g |
VIP2Y
|
BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD.
|
2024-10-11 | |
| $10.00/1KG |
VIP5Y
|
Hebei Chuanghai Biotechnology Co,.LTD
|
2024-08-14 | |
| $130.00/100mg |
VIP3Y
|
Zibo Hangyu Biotechnology Development Co., Ltd
|
2024-07-10 |
- Since: 2020-08-05
- Address: 804, Unit 2, Building 4, i City, No. 11, South Tangyan Road, High-tech Zone, Xi 'an, Shaanxi











 China
China