
Product Details
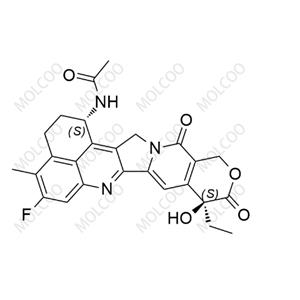
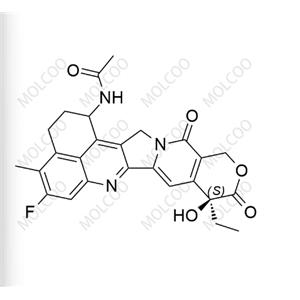
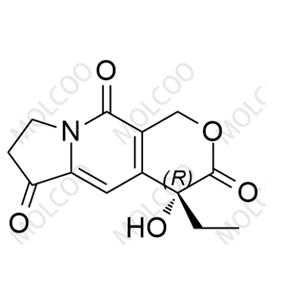
| Product Name: Exatecan Impurity2 | CAS No.: 143655-59-8 |
| Min. Order: 10mg | Purity: 99%+ HPLC |
| Supply Ability: 1000 | Release date: 2025/07/31 |
Exatecan Impurity

Company Profile Introduction
-
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/10mg |
VIP8Y
|
Guangzhou PI PI BIOTECH INC
|
2020-06-18 | |
| $0.00/1KG |
VIP6Y
|
Shaanxi Dideu Medichem Co. Ltd
|
2025-09-11 | |
| $2.00/100kg |
VIP1Y
|
ZHENGZHOU JIUYI TIME NEW MATERIALS CO,.LTD
|
2025-06-18 |
INQUIRY









 China
China