
Hexachloroethane NEW
| Price | Get Latest Price |
| Package | 25KG |
| Min. Order: | 1KG |
| Supply Ability: | 50000KG/month |
| Update Time: | 2023-08-29 |
Product Details
| Product Name: Hexachloroethane | CAS No.: 67-72-1 |
| EC-No.: 200-666-4 | Min. Order: 1KG |
| Purity: 99% | Supply Ability: 50000KG/month |
| Release date: 2023/08/29 |
| CAS: | 67-72-1 |
| MF: | C2Cl6 |
| MW: | 236.74 |
| EINECS: | 200-666-4 |
| Product Categories: | API Intermediate;Alpha Sort;E-LAlphabetic;H;HA -HT;Volatiles/ Semivolatiles;refrigerants;Organics;67-72-1 |
| Mol File: | 67-72-1.mol |
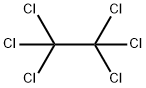 | |
| Hexachloroethane Chemical Properties |
| Melting point | 183-185 °C (dec.) (lit.) |
| Boiling point | 186℃ |
| density | 2.091 g/mL at 25 °C (lit.) |
| vapor density | 8.16 (vs air) |
| vapor pressure | 0.4 mm Hg ( 20 °C) |
| refractive index | 1.5282 (estimate) |
| Fp | 9℃ |
| storage temp. | 2-8°C |
| solubility | Soluble in alcohol, benzene, chloroform, ether |
| form | Crystals or Crystalline Powder |
| color | White |
| Water Solubility | 0.05 g/L (22 ºC) |
| Merck | 14,4679 |
| BRN | 1740341 |
| Henry's Law Constant | 1.43, 2.81, and 5.31 at 10, 20, and 30 °C, respectively (Munz and Roberts, 1987) |
| Exposure limits | TLV-TWA 10 ppm (~100 mg/m3) (ACGIH), 1 ppm (MSHA and OSHA), Lowest Feasi ble Limit (NIOSH); carcinogenicity: Animal Limited Evidence (IARC). |
| Stability: | Stable. Non-combustible. May react with hot metals, strong oxidizing agents. |
| CAS DataBase Reference | 67-72-1(CAS DataBase Reference) |
| IARC | 2B (Vol. 73) 1999 |
| NIST Chemistry Reference | Ethane, hexachloro-(67-72-1) |
| EPA Substance Registry System | Hexachloroethane (67-72-1) |
| Hexachloroethane Usage And Synthesis |
| Overview | Hexachloroethane (HCE; CASRN 67-72-1) is a halogenated hydrocarbon consisting of six chlorines attached to an ethane backbone. In the past, HCE was used as an anti-helminthic for the treatment of sheep flukes, but is no longer used for this purpose since the U.S. Food and Drug Administration (FDA) withdrew approval for this use in 1971[1]. HCE is primarily used by the military for smoke pots; smoke grenades, and pyrotechnic devices[1]. HCE has also been used as a polymer additive, a moth repellant, a plasticizer for cellulose esters, and an insecticide solvent, and in metallurgy for refining aluminum alloys[1, 3]. HCE was also identified in the headspace of chlorine-bleach-containing household products[4].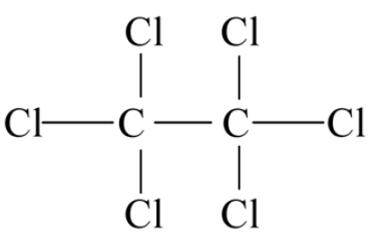 Figure 1 the chemical structure of hexachloroethane HCE is produced by the chlorination of tetrachloroethylene (PERC) in the presence of ferric chloride[1]. HCE was produced in the United States (U.S.) for commercial distribution from 1921 to 1967, but is currently not commercially distributed[1, 5]. In the 1970s, U.S. producers of HCE reported that HCE was not distributed, but was only used in-house or recycled[1]; U.S. distributors in the 1970s imported HCE from France, Spain, and the United Kingdom[1]. U.S. production plus imports of HCE totaled 10 million–50 million pounds in 1986, 1 million–10 million pounds in 1990, 10 million–50 million pounds in 1994, 500,000–1 million pounds in 1998, 10,000–500,000 pounds in 2002, and 1–10 million pounds in 2006[6]. |
| Application | Hexachloroethane was reported to be used as a chemical intermediate, as a flux agent for grain refining and degassing of aluminum alloys, and as a flame retardant in industrial laminating resins. It was also reported to be used as a reactant in military smoke ammunition. Other uses of hexachloroethane noted in earlier scientific and technical literature were in military pyrotechnics, in the metallurgical industry, as a plasticizer, as an ignition suppressant, as a processing aid in various industrial processes, as a component of fungicidal and insecticidal formulations, and (formerly) as an anthelmintic in veterinary medicine[1, 7]. The use of hexachloroethane in cosmetics appears on the List of Prohibited and Restricted Cosmetic Ingredients (more commonly referred to as the Cosmetic Ingredient Hotlist or simply the Hotlist), an administrative tool that Health Western countries uses to communicate to manufacturers and others that certain substances, when present in a cosmetic, may contravene (a) the general prohibition found in section 16 of the Food and Drugs Act or (b) a provision of the Cosmetic Regulations. Hexachloroethane is not used in pesticide formulations in Western countries. Also, currently in Western countries, hexachloroethane is not present in veterinary products, it is no longer used in military smoke ammunition, and no evidence has been found for its current use as a flame retardant. Hexachloroethane is not an approved food additive in western countries and was not present in various regulatory food databases[8, 9]. It does however continue to be imported into Western countries for use as a degassing agent for oxides and hydrogen elimination from aluminum alloys during die casting at a quantity of less than 2000 kg per year. The production and uses of hexachloroethane are being phased out internationally. The European Commission prohibits the use of hexachloroethane in the manufacturing or processing of non-ferrous metals[10]. In the United States, there has been a trend away from using hexachloroethane flux in the secondary aluminum industry[11]. Similarly, representatives of the aluminum industry in the United States report that hexachloroethane is no longer used in most primary aluminum degassing[12]. The Aluminum Association of western countries has also reported that its members do not use hexachloroethane in their activities (primary aluminum industry). It was reported that hexachloroethane may be a constituent of lubricating greases and oils, non-structural caulking compounds and sealants, automotive chemicals; laundry and ironing aids and dry cleaning agents, but no quantitative data were provided[13]. |
| Environmental fate | Hexachloroethane is an industrial chemical that is not known to occur naturally. It is not produced for commercial distribution in the United States, but is imported for use in military smoke and pyrotechnic devices and as an intermediate in the organic chemicals industry. It is released to the environment from these uses, primarily to the atmosphere. Hexachloroethane is relatively persistent in the environment. It volatilizes readily from water to the atmosphere, with a half-life of less than one day in some waters. Hexachloroethane may also leach through soil to groundwater. Neither hydrolysis nor photolysis is expected to be important removal processes, but hexachloroethane may be reduced in aquatic systems in the presence of specific agents. Bioconcentration in fish has been reported, but bio magnification through the food chain is unlikely. Biodegradation may contribute to hexachloroethane removal from ambient waters, but there is conflicting evidence regarding the significance of this fate process for hexachloroethane. Hexachloroethane has been detected at low (ng/m3) levels in the atmosphere and occasionally in drinking water systems. It is rarely detected in surface waters or biota, and has not been reported in ambient soil, sediments, or commercial food products. Hexachloroethane has been identified in at least 45 of the 1,416 hazardous waste sites that have been proposed for inclusion on the EPA National Priorities List (NPL) (HazDat 1995). However, the number of sites evaluated for hexachloroethane is not known. |
| Health effect | Mild skin irritation can occur when workers at a munitions factory were exposed to low levels of hexachloroethane[15]. The workers should wear protective clothing to greatly reduce exposure. Based on the animal data, hexachloroethane in the air can irritate human's nose and lungs and cause some buildup of mucus in the nose, much like an allergy. It can also irritate the eyes and make them tear. People in an area having a lot of hexachloroethane vapor may have their facial muscles twitch or have difficulty moving[15]. These effects have been observed in animals during exposure at levels far greater than those found in industrial use of hexachloroethane or those that would be expected in areas near a hazardous waste site. Hexachloroethane is not a highly toxic substance. People who exposed to a large amount for a long time may have their liver cells destroyed and fat built up in your liver. There is also a slight chance that the kidneys could be damaged[15]. Although no results from animal studies suggest that hexachloroethane would make it hard for you to become pregnant or that it would hurt your baby while you are pregnant, animal studies that have looked at the effects of hexachloroethane during pregnancy are limited[15]. Liver tumors can develop in mice that were orally exposed to hexachloroethane for their whole lifetime. Liver tumors are common in mice. Hexachloroethane may not necessarily have the same effect on people. Male rats that were exposed to hexachloroethane for their lifetime developed kidney tumors. This type of tumor is not found in people, so it is unlikely that exposure to hexachloroethane would cause you to develop cancer of the kidney. The Department of Health and Human Services claims that hexachloroethane may reasonably be anticipated to be a carcinogen (can cause cancer). The International Agency for Research on Cancer (IARC) has determined that hexachloroethane is not classifiable as to its carcinogenicity in people. EPA has determined that hexachloroethane is a possible human carcinogen[15]. |
| Toxico-kinetics | No studies have evaluated HCE absorption in humans by oral or inhalation exposure. HCE was identified in follicular fluid of women undergoing in vitro fertilization (IVF) during an analysis for environmental contaminants[16]. These data indicate the potential for HCE uptake, but not the source or route of exposure. The dermal absorption rate of HCE has been described as limited[1]; the absorption of a saturated HCE solution across human skin was estimated to be 0.023 mg/cm2•hour[17]. There are limited data on the distribution of HCE in humans[16]. Animal studies have consistently demonstrated that HCE is distributed to fat, kidney, liver, and blood[18, 19]. Data from in vivo and in vitro studies support a conclusion that metabolism of HCE is incomplete, with excretion of unmetabolized HCE in exhaled air and possibly in urine. In vivo metabolism data for HCE are limited to three studies: Mitoma et al. (1985) in rats and mice[20]; Jondorf et al. (1957) in rabbits[21]; and Fowler (1969) in sheep[22]. Each of these studies suggest limited metabolism for HCE. A variety of intermediary metabolites have also been identified in exhaled air and urine[21, 22]. In vitro studies using liver microsomes indicated that HCE metabolism involves phenobarbital-inducible cytochrome P450 (CYP450) enzymes[23, 24]; however, no specific enzymes have been identified. The CYP450 enzymes induced by phenobarbital include those from the 2A, 2B, 2C, and 3A subfamilies. One study[25] found evidence for CYP1A2 involvement in the metabolism of HCE, although this was not supported by the results from in vitro studies with 3-methylcholanthrene, an inducer of the CYP450 1 subfamily[23, 24]. No available studies evaluated the HCE elimination in humans. Animal studies indicated that the major routes of HCE elimination are either by fecal matter or by expired air[20-22]. Sheep studies[22] indicated that orally administered HCE is eliminated by the fecal route without absorption and metabolism, while rodent studies[20] provided evidence that HCE is absorbed and eliminated by exhalation. It is unknown why there is a difference in elimination between sheep and rodents. |
| References |
|
| Description | Hexachloroethane (HCE) is a halogenated hydrocarbon consisting of six chlorines attached to an ethane (ACGIH, 1991); it is a white to pale yellow solid that is unstable in air and evaporates gradually. It smells like camphor when its concentration in air and water are 150 and 10 ppb, respectively. HCE itself does not catch fire easily; however; in aqueous nonbiological conditions it has been determined that HCE is unstable and nonenzymatic dechlorination in the absence of nicotinamide adenine dinucleotide phosphate (NADP) occurs. It rapidly degrades in soil or groundwater. Also, some microorganisms break down HCE without oxygen, and decomposition in aerobic conditions has been reported. Some bioconcentration of HCE in fish has been determined, though upper levels through the food chain are limited, since it is rapidly metabolized by fish, which is discussed later (ATSDR, 1997). Eyes, skin, respiratory system, and kidneys have been proposed as main targets in humans upon exposure. Symptoms include blinking, tearing, photophobia, and irritation of eyes. Also, facial muscles may have difficulty in movement. Animal studies on effects of HCE during pregnancy are limited. After oral exposure, HCE is primarily distributed to fat tissue. Toxicokinetic studies in animals indicated that HCE is mostly localized and metabolized in the liver and kidney. Several corresponded metabolites have demonstrated liver and kidney toxicities similar to HCE. Neurological effects such as tremors and ataxia were observed in Beagle dogs, rats, and pregnant rats. Other effects via inhalation exposure included reduced body weight and increased relative liver weight in rats and guinea pigs. In another study, male rats also displayed increased relative spleen and testes weight. Based on California Proposition 65, HCE was proposed to be carcinogenic for humans, and it induces tumors at sites other than the site of entry. Noncancerous effects include kidney degeneration (tubular nephropathy, necrosis of renal tubular epithelium, hyaline droplet formation, tubular regeneration, and tubular casts) and hepatocellular necrosis. It results in hyaline droplet nephropathy and renal toxicity, and it induces chromosome malsegregation, lethality, and mitotic growth arrest (Crebelli et al., 1995, 1992, 1988). |
| Chemical Properties | white crystalline powder |
| Chemical Properties | Hexachloroethane is a white solid with a camphor-like odor. It gradually evaporates when it is exposed to air. |
| Physical properties | Rhombic, triclinic or cubic, colorless crystals with a camphor-like odor. Odor threshold concentration is 0.15 ppm (quoted, Amoore and Hautala, 1983). |
| Uses | Hexachloroethane is used as a solvent, infireworks and smoke devices; in explosives,in celluloid, as an insecticide, and as a rubbervulcanizing accelerator. Earlier it was used asan anthelmintic for livestock. Hexachloroethane is a highly efficient chlorinating agent in the preparation of chlorosilanes from hydrosilanes. |
| Uses | In metallurgy for refining aluminum alloys, removing impurities from molten metals, recovering metal from ores or smelting products. Degassing agent for magnesium; to inhibit explosiveness of methane and combustion of ammonium perchlorate. Smoke generator in grenades; in pyrotechnics. Ignition suppressant, in fire extinguishing fluids, polymer additive, flame-proofing agent, vulcanizing agent. In production of synthetic diamonds. |
| Uses | The applications of hexachloroethane have been extensive; however, industrial uses are diminishing. Hexachloroethane is used primarily in military smoke munitions (e.g., smoke pots, grenades, cartridges, and projectiles used to generate “smoke” or “fog”) and in pyrotechnics. The estimated average annual use of hexachloroethane from 1966 to 1977 at a major facility manufacturing smoke and pyrotechnic devices was 192,802 lb. In the 1970s, about half of the hexachloroethane distributed was used to manufacture military smoke-producing and pyrotechnic devices, 30% to 40% to manufacture degassing pellets to remove air bubbles from molten ore at aluminum foundries, and 10% to 20% as an antihelminthic to control liver flukes in sheep and cattle. The U.S. Food and Drug Administration withdrew approval for the use of hexachloroethane as an antihelminthic in 1971, and it probably is no longer used for this purpose (ATSDR 1997). Its use for degassing aluminum also has been almost completely phased out in the United States (EPA 1999). Other uses in metallurgy include refining alloys, removing impurities from molten metals, recovering metals from ores or smelting products, and as a degassing agent for magnesium; however, the European Union began phasing out the use of hexachloroethane in nonferrous metals in 1998 (EC 1998). A number of other past uses of hexachloroethane have been identified, but many of these likely have been discontinued or involve the use of only limited quantities. Hexachloroethane is used as a laboratory chemical and as an ingredient in various fungicidal and insecticidal formulations, extreme-pressure lubricants, and plastics (ATSDR 1997, IARC 1999, HSDB 2009). Other past uses include as a moth repellent and in the chemical industry as a polymer additive, a plasticizer for cellulose esters, an accelerator, a vulcanizing agent, a process solvent in rubber manufacturing, a retardant in fermentation processes, and a component of submarine paints, and in the production of some types of synthetic diamonds. It has also been used as a component of fire-extinguishing fluids, an additive in combustible liquids (ignition suppressant), and an inhibitor of the explosiveness of methane and the combustion of ammonium perchlorate (IARC 1979, 1999, HSDB 2009). |
| Definition | ChEBI: A member of the class of chloroethanes that is ethane in which all the hydrogens are replaced by chloro groups. |
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $50.00/1KG |
VIP1Y
|
Henan Fengda Chemical Co., Ltd
|
2023-12-22 | |
| $5.00/1KG |
VIP3Y
|
Hebei Guanlang Biotechnology Co,.LTD
|
2023-03-08 | |
| $0.00/25KG |
VIP2Y
|
Hebei Guanlang Biotechnology Co., Ltd.
|
2023-01-06 | |
| $1.50/1g |
VIP2Y
|
Shaanxi Didu New Materials Co. Ltd
|
2022-05-20 | |
| $10.00/1KG |
Hebei baicao biology science and technology co., ltd
|
2022-04-15 | ||
| $9.90/1KG |
VIP6Y
|
Hebei Guanlang Biotechnology Co., Ltd.
|
2021-10-08 | |
| $0.00/100KG |
Shaanxi Dayu Chemical Co., Ltd
|
2020-06-17 | ||
| $0.10/1KG |
VIP5Y
|
Shaanxi Dideu Medichem Co. Ltd
|
2020-01-08 | |
| $1.00/1KG |
VIP6Y
|
Career Henan Chemical Co
|
2018-08-20 |
- Since: 2017-12-08
- Address: Building A, Enjoy city, Zhongshan East Road, Shijiazhuang city, Hebei province
13288715578
sales@hbmojin.com










 China
China