
PENTADECANOIC ACID NEW
| Price | Get Latest Price |
| Package | 25KG |
| Min. Order: | 1KG |
| Supply Ability: | 50000KG/month |
| Update Time: | 2023-10-26 |
Product Details
| Product Name: PENTADECANOIC ACID | CAS No.: 1002-84-2 |
| EC-No.: 213-693-1 | Min. Order: 1KG |
| Purity: 99% | Supply Ability: 50000KG/month |
| Release date: 2023/10/26 |
| CAS: | 1002-84-2 |
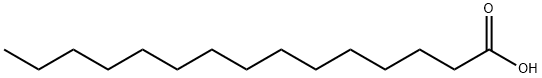
| MF: | C15H30O2 |
| MW: | 242.4 |
| EINECS: | 213-693-1 |
| Product Categories: | Alkylcarboxylic Acids;Biochemistry;Higher Fatty Acids & Higher Alcohols;Monofunctional & alpha,omega-Bifunctional Alkanes;Monofunctional Alkanes;Saturated Higher Fatty Acids;C13 to C42+Fatty Acids;PA - PENFatty Acids;Fatty AcidsFA/FAME/Lipids/Steroids;Free Fatty Acids;Lipid Analytical Standards;NeatsAlphabetic;Other Lipid Related Products;P;Fatty Acids;Saturated fatty acids and derivatives;Straight chain fatty acids;Carbonyl Compounds;Carboxylic Acids |
| Mol File: | 1002-84-2.mol |
 | |
| PENTADECANOIC ACID Chemical Properties |
| Melting point | 51-53 °C(lit.) |
| Boiling point | 257 °C100 mm Hg(lit.) |
| density | 0.8423 |
| FEMA | 4334 | PENTADECANOIC ACID |
| refractive index | 1.4292 |
| Fp | >230 °F |
| storage temp. | Store below +30°C. |
| solubility | Soluble in ethanol. |
| pka | 4.78±0.10(Predicted) |
| form | Powder or Flakes |
| color | White |
| Odor | waxy |
| Water Solubility | 12mg/L(20 ºC) |
| BRN | 1773831 |
| Stability: | Stable. Combustible. Incompatible with bases, reducing agents, oxidizing agents. |
| LogP | 6.62 |
| CAS DataBase Reference | 1002-84-2(CAS DataBase Reference) |
| EPA Substance Registry System | Pentadecanoic acid (1002-84-2) |
| Applications | The majority of research into fatty acid metabolism has been conducted primarily on even chain fatty acids (carbon chain length of 2–26) as these represent >99% of the total fatty acid plasma concentration in humans[13,14]. However, there is also a detectable amount of odd-chain fatty acids in human tissue. As a result of the low concentration there are only four significantly measureable odd chain fatty acids, which are C15:0, C17:0, C17:1[25] and C23:0[26]. C15:0 and C17:0; these have been gaining research interest within the scientific community as they have been found to be important as: (1) quantitative internal standards; (2) biomarkers for dietary food intake assessment; (3) biomarkers for coronary heart disease (CHD) risk and type II diabetes mellitus (T2D) risk (although the objective is not to provide a meta-analysis of odd chain saturated fatty acids (OCS-FAs) and disease risk); (4) evidence for theories of alternate endogenous metabolic pathways. Quantitative internal standards Since the early 1960s, it has been concluded that odd chain saturated fatty acids (OCS-FAs) are of little physiological significance[27–29] and that the only real difference with their more abundant counterparts, even chain fatty acids[24], is seen in the endpoint of metabolism where OCS-FAs result in propionyl CoA[29] as opposed to acetyl CoA[30]. Moreover, the OCS-FAs are present at apparently insignificant plasma concentrations[31] (<0.5% total plasma fatty acid concentration[32]) and the natural variation of concentrations within blood plasma ranging from 0%–1%. Therefore, OCS-FAs can be used as low cost internal standards in quantitative analysis, with C15:0 fatty acids being the most widely employed in this context. Many assumed that the concentration of OCS-FAs did not vary in different diseases and these lipid species were commonly used for standards in analyses[33,34]. The natural plasma variation of C15:0 could account for a 0.2%–3% variation in the Q-Int. Std response and therefore affecting the observed instrument abundance of the analytes. Furthermore, the use of these two OCS-FAs as quantitative internal standards does not allow them to be incorporated into any statistical analysis and therefore no correlations can be deduced. This is the main limiting factor to the amount of understand there is around the physiology of OCS-FAs. Biomarkers for dietary food intake assessment With the realization that OCS-FAs are in fact a biologically relevant component of blood plasma[35] there came further insights into their origin, either through consumption or through endogenous biosynthetic or metabolic pathways. This new direction of research interest led into the field of dietary analysis and the aim to identify lipidome variations[36] in relation to dietary intake[37]. OCS-FAs have attracted attention with research into the possible application of C15:0 in blood as a marker for intake of milk fat and subsequent relations between intake of milk fat with metabolic risk factors, the results in the first published study that focused on this showed that the proportions of C15:0 in cholesterol esters are associated with the total amount of fat from milk products (r = 0.46, p < 0.0001), based on 62 men[46]. Biomarkers for coronary heart disease (CHD) risk and type II diabetes mellitus (T2D) risk In recent years, researches has been carried out in two key studies: The European Prospective Investigation into Cancer and Nutrition (EPIC) and The Norfolk Prospective Study[38]. The plasma samples of 1595 CHD cases and 2246 controls were used to extract plasma phospholipid fatty acids. The lipid extracts were measured by gas chromatography coupled to electron impact mass spectrometry and the concentrations were determined by peak comparison with an internal standard (di-palmitoyl-D31-phosphatidylcholine). The incidence of CHD was ascertained by the participant’s admission into hospital with a CHD diagnosis or death from CHD according to ICD9 410-414/ICD10 I22–I25. The results from this study clearly revealed saturated plasma phospholipid fatty acid, C14:0, C16:0, C18:0, concentrations were significantly associated with an increased risk of CHD. However, OCS-FAs concentrations of C15:0 and C17:0 showed a significant inverse association with CHD incidence, making them potential biomarkers of CHD. |
| Description | Pentadecanoic acid is a saturated fatty acid. Its molecular formula is CH3(CH2)13COOH. It is rare in nature, being found at the level of 1.2 % in the milk fat from cows . The butterfat in cows milk is its major dietary source and it is used as a marker for butterfat consumption. Pentadecanoic acid also occurs in hydrogenated mutton fat. Pentadecanoic acid may increase mother-to-child transmission of HIV through breastfeeding. |
| Chemical Properties | White solid; waxy aroma. |
| Chemical Properties | white powder |
| Occurrence | Reported found in Herniaria incana lam. oil Greece (0.30%), Glycosmis pentaphylla (cor.) bark oil India (0.20%), Thevetia peruviana (pers.) K. Schum. flower oil Brazil (0.20%), and thyme oil Spain (0.10%). |
| Uses | Pentadecanoic acid is a saturated fatty acid. Pentadecanoic acid was utilized as a biomarker to examine for the intake of milk fat in relation to its metabolic risk factors. Pentadecanoic Acid that is also produced by certain plant species and acts as an toxic essential oil, which is known to exhibits potential anxiolytic, antinociceptive and antimicrobial properties. |
| Uses | Pentadecanoic acid is a saturated fatty acid. Pentadecanoic acid was utilized as a biomarker to examine for the intake of milk fat in relation to its metabolic risk factors. Pentadecanoic Acid that is also produced by certain plant species and acts as an toxic essential oil, which is known to exhibits potential anxiolytic, antinociceptive and antimicrobial properties. |
| Definition | ChEBI: Pentadecanoic acid is a straight-chain saturated fatty acid containing fifteen-carbon atoms. It has a role as a plant metabolite, a food component, a Daphnia magna metabolite, a human blood serum metabolite and an algal metabolite. It is a long-chain fatty acid and a straight-chain saturated fatty acid. It is a conjugate acid of a pentadecanoate. |
| Aroma threshold values | Medium strength odor |
| Synthesis Reference(s) | Tetrahedron Letters, 24, p. 4993, 1983 DOI: 10.1016/S0040-4039(01)99830-2 |
| General Description | Pentadecanoic acid is a saturated fatty acid, commonly present in ox bile. It is present as a phytochemical component of Indigofera suffruticosa leaves and is known to exhibit antimicrobial and antioxidant properties. |
| Safety Profile | Poison by intravenous route. When heated to decomposition it emits acrid smoke and irritating fumes. |
| Purification Methods | Crystallise the acid from Et2O and distil it in vacuo. It is very hygroscopic. See the purification of palmitic acid. [Beilstein 2 IV 1147.] |
| Applications | The majority of research into fatty acid metabolism has been conducted primarily on even chain fatty acids (carbon chain length of 2–26) as these represent >99% of the total fatty acid plasma concentration in humans[13,14]. However, there is also a detectable amount of odd-chain fatty acids in human tissue. As a result of the low concentration there are only four significantly measureable odd chain fatty acids, which are C15:0, C17:0, C17:1[25] and C23:0[26]. C15:0 and C17:0; these have been gaining research interest within the scientific community as they have been found to be important as: (1) quantitative internal standards; (2) biomarkers for dietary food intake assessment; (3) biomarkers for coronary heart disease (CHD) risk and type II diabetes mellitus (T2D) risk (although the objective is not to provide a meta-analysis of odd chain saturated fatty acids (OCS-FAs) and disease risk); (4) evidence for theories of alternate endogenous metabolic pathways. Quantitative internal standards Since the early 1960s, it has been concluded that odd chain saturated fatty acids (OCS-FAs) are of little physiological significance[27–29] and that the only real difference with their more abundant counterparts, even chain fatty acids[24], is seen in the endpoint of metabolism where OCS-FAs result in propionyl CoA[29] as opposed to acetyl CoA[30]. Moreover, the OCS-FAs are present at apparently insignificant plasma concentrations[31] (<0.5% total plasma fatty acid concentration[32]) and the natural variation of concentrations within blood plasma ranging from 0%–1%. Therefore, OCS-FAs can be used as low cost internal standards in quantitative analysis, with C15:0 fatty acids being the most widely employed in this context. Many assumed that the concentration of OCS-FAs did not vary in different diseases and these lipid species were commonly used for standards in analyses[33,34]. The natural plasma variation of C15:0 could account for a 0.2%–3% variation in the Q-Int. Std response and therefore affecting the observed instrument abundance of the analytes. Furthermore, the use of these two OCS-FAs as quantitative internal standards does not allow them to be incorporated into any statistical analysis and therefore no correlations can be deduced. This is the main limiting factor to the amount of understand there is around the physiology of OCS-FAs. Biomarkers for dietary food intake assessment With the realization that OCS-FAs are in fact a biologically relevant component of blood plasma[35] there came further insights into their origin, either through consumption or through endogenous biosynthetic or metabolic pathways. This new direction of research interest led into the field of dietary analysis and the aim to identify lipidome variations[36] in relation to dietary intake[37]. OCS-FAs have attracted attention with research into the possible application of C15:0 in blood as a marker for intake of milk fat and subsequent relations between intake of milk fat with metabolic risk factors, the results in the first published study that focused on this showed that the proportions of C15:0 in cholesterol esters are associated with the total amount of fat from milk products (r = 0.46, p < 0.0001), based on 62 men[46]. Biomarkers for coronary heart disease (CHD) risk and type II diabetes mellitus (T2D) risk In recent years, researches has been carried out in two key studies: The European Prospective Investigation into Cancer and Nutrition (EPIC) and The Norfolk Prospective Study[38]. The plasma samples of 1595 CHD cases and 2246 controls were used to extract plasma phospholipid fatty acids. The lipid extracts were measured by gas chromatography coupled to electron impact mass spectrometry and the concentrations were determined by peak comparison with an internal standard (di-palmitoyl-D31-phosphatidylcholine). The incidence of CHD was ascertained by the participant’s admission into hospital with a CHD diagnosis or death from CHD according to ICD9 410-414/ICD10 I22–I25. The results from this study clearly revealed saturated plasma phospholipid fatty acid, C14:0, C16:0, C18:0, concentrations were significantly associated with an increased risk of CHD. However, OCS-FAs concentrations of C15:0 and C17:0 showed a significant inverse association with CHD incidence, making them potential biomarkers of CHD. |
| References |
|
| Description | Pentadecanoic acid is a saturated fatty acid. Its molecular formula is CH3(CH2)13COOH. It is rare in nature, being found at the level of 1.2 % in the milk fat from cows . The butterfat in cows milk is its major dietary source and it is used as a marker for butterfat consumption. Pentadecanoic acid also occurs in hydrogenated mutton fat. Pentadecanoic acid may increase mother-to-child transmission of HIV through breastfeeding. |
| Chemical Properties | White solid; waxy aroma. |
| Chemical Properties | white powder |
| Occurrence | Reported found in Herniaria incana lam. oil Greece (0.30%), Glycosmis pentaphylla (cor.) bark oil India (0.20%), Thevetia peruviana (pers.) K. Schum. flower oil Brazil (0.20%), and thyme oil Spain (0.10%). |
| Uses | Pentadecanoic acid is a saturated fatty acid. Pentadecanoic acid was utilized as a biomarker to examine for the intake of milk fat in relation to its metabolic risk factors. Pentadecanoic Acid that is also produced by certain plant species and acts as an toxic essential oil, which is known to exhibits potential anxiolytic, antinociceptive and antimicrobial properties. |
| Uses | Pentadecanoic acid is a saturated fatty acid. Pentadecanoic acid was utilized as a biomarker to examine for the intake of milk fat in relation to its metabolic risk factors. Pentadecanoic Acid that is also produced by certain plant species and acts as an toxic essential oil, which is known to exhibits potential anxiolytic, antinociceptive and antimicrobial properties. |
| Definition | ChEBI: Pentadecanoic acid is a straight-chain saturated fatty acid containing fifteen-carbon atoms. It has a role as a plant metabolite, a food component, a Daphnia magna metabolite, a human blood serum metabolite and an algal metabolite. It is a long-chain fatty acid and a straight-chain saturated fatty acid. It is a conjugate acid of a pentadecanoate. |
| Aroma threshold values | Medium strength odor |
| Synthesis Reference(s) | Tetrahedron Letters, 24, p. 4993, 1983 DOI: 10.1016/S0040-4039(01)99830-2 |
| General Description | Pentadecanoic acid is a saturated fatty acid, commonly present in ox bile. It is present as a phytochemical component of Indigofera suffruticosa leaves and is known to exhibit antimicrobial and antioxidant properties. |
| Safety Profile | Poison by intravenous route. When heated to decomposition it emits acrid smoke and irritating fumes. |
| Purification Methods | Crystallise the acid from Et2O and distil it in vacuo. It is very hygroscopic. See the purification of palmitic acid. [Beilstein 2 IV 1147.] |
Packing &shipping&Payment
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $6.00/1KG |
VIP1Y
|
Hebei Longbang Technology Co., Ltd
|
2024-04-28 | |
| $6.00/1KG |
VIP1Y
|
Hebei Saisier Technology Co., LTD
|
2024-04-17 | |
| $200.00/1KG |
VIP1Y
|
Henan Fengda Chemical Co., Ltd
|
2023-12-30 | |
| $0.00/1kg |
VIP1Y
|
Hebei Jingbo New Material Technology Co., Ltd
|
2023-12-22 | |
| $40.00/1kg |
VIP4Y
|
Hebei Yanxi Chemical Co., Ltd.
|
2023-10-16 | |
| $0.00/25KG |
PNP Biotech Co. Ltd
|
2023-04-28 | ||
| $0.00/25KG |
VIP2Y
|
Hebei Guanlang Biotechnology Co., Ltd.
|
2022-11-14 | |
| $0.00/1kg |
BTC Nantong Pharmaceuticals Technology Co.,Ltd
|
2022-08-22 | ||
| $500.00/1KG |
VIP6Y
|
Career Henan Chemical Co
|
2018-08-04 |
- Since: 2017-12-08
- Address: Building A, Enjoy city, Zhongshan East Road, Shijiazhuang city, Hebei province
13288715578
sales@hbmojin.com











 China
China