
Pramipexole NEW
| Price | Get Latest Price |
| Package | 25KG |
| Min. Order: | 1KG |
| Supply Ability: | 50000KG/month |
| Update Time: | 2023-08-29 |
Product Details
| Product Name: Pramipexole | CAS No.: 104632-26-0 |
| EC-No.: 600-593-1 | Min. Order: 1KG |
| Purity: 99% | Supply Ability: 50000KG/month |
| Release date: 2023/08/29 |
| CAS: | 104632-26-0 |
| MF: | C10H17N3S |
| MW: | 211.33 |
| EINECS: | 600-593-1 |
| Product Categories: | Mirapexin, Sifrol;API;All Inhibitors;Inhibitors;Chiral Reagents;Sulfur & Selenium Compounds;Intermediates & Fine Chemicals;Pharmaceuticals |
| Mol File: | 104632-26-0.mol |
 | |
| Pramipexole Chemical Properties |
| Melting point | 288-290°C |
| Boiling point | 378.0±42.0 °C(Predicted) |
| density | 1.17±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | 9.47±0.20(Predicted) |
| form | Solid |
| color | White to Off-White |
| CAS DataBase Reference | 104632-26-0(CAS DataBase Reference) |
| Pramipexole Usage And Synthesis |
| Chemical Properties | Off-White Solid |
| Originator | Mirapex, Pharmacia and Upjohn, USA |
| Uses | (S)-Pramipexole enantiomer. A dopamine-D2-receptor agonist. Antiparkinsonian |
| Uses | partial/full D2S, D2L, D3, D4 receptor agonist |
| Uses | (S)-Pramipexole enantiomer. s disease (PD) and restless legs syndrome (RLS).[1] It is also sometimes used off-label as a treatment for cluster headache and to counteract the problems with sexual dysfunction experienced by some u sers of the selective serotonin reuptake inhibitor (SSRI) antidepressants. |
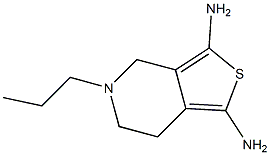
| Definition | ChEBI: A member of the class of benzothiazoles that is 4,5,6,7-tetrahydro-1,3-benzothiazole in which the hydrogens at the 2 and 6-pro-S-positions are substituted by amino and propylamino groups, respectively. |
| Manufacturing Process | 75.5 g (0.5 mol) of 4-aminocyclohexanol hydrochloride and 74.0 g (0.5 mol) of phthalic acid anhydride are mixed with 65 g (0.5 mol) of ethyldiisopropyl amine and 1000 ml of toluene and boiled for 36 hours with a water separator. Then water is added, the toluene phase is separated off and the aqueous phase is extracted several times with chloroform. The organic phases are combined, dried and concentrated. The concentrated residue is recrystallised from isopropanol and 4-(phthalimido)-cyclohexanol was obtained. Yield: 95 g (77.8%). Melting point 175°-176°C. 95 g (0.388 mol) of 4-(phthalimido)-cyclohexanol are dissolved in 600 ml of chloroform and, after the addition of 450 ml of water and 120 ml of sulfuric acid, 90 g (0.3 mol) of potassium dichromate are added in batches. The internal temperature of the mixture is maintained at between 25° and 30°C by slight cooling. The mixture is stirred for a further 3 hours, then the chloroform phase is separated off and the mixture extracted twice more with chloroform. After drying and concentration of the extracts 82 g (86.9%) of 4(phthalimido)-cyclohexanone was obtained. 48.6 g (0.2 mol) of 4-(phthalimido)cyclohexanone are dissolved in glacial acetic acid, mixed with 36% of hydrobromic acid in glacial acetic acid and then 32 g (0.2 mol) of bromine in glacial acetic acid is added dropwise with cooling. The mixture is then concentrated by evaporation in vacuo and the residue is triturated several times with diethylether. The ether extracts are discarded and the residue is dissolved in of ethanol. After thiourea have been added the mixture is refluxed for 5 hours. It is then concentrated by evaporation, made alkaline with sodium hydroxide solution and extracted with chloroform. After drying and concentration of the extracts, the residue is purified by column chromatography on silica gel (eluant: chloroform/methanol = 1/1). The 2-amino-6-phthalimido-4,5,6,7-tetrahydro-benzthiazol was obtained. Melting point 244-246°C, dec. Yield: 30 g (50%). 9.5 g (31.7 mmol) of 2-amino-6-phthalimido-4,5,6,7-tetrahydro-benzthiazole are suspended in 100 ml of ethanol and, after the addition of 1.8 g (36 mmol) of hydrazine hydrate, refluxed for 2 hours. The mixture is then concentrated and purified by column chromatography on silica gel using methanol as eluant. The 2,6-diamino- 4,5,6,7-tetrahydro-benzthiazole was obtained. To a solution of 2,6-diamino- 4,5,6,7-tetrahydro-benzthiazole in dimethylformamide are added n-propanal and the mixture is heated to 50°C for 1 hour. After cooling, the reaction solution is mixed with sodium borohydride and heated to 50°C for 30 min. The solvent is largely eliminated in vacuo. Whilst cooling with ice, the residue is mixed with water and 2 N hydrochloric acid until a pH of 1 is obtained. The aqueous solution is exwith ethylacetate and the organic phase discarded. The aqueous phase is mixed with potassium carbonate until an alkaline reaction is obtained and then extracted with ethyl acetate. The organic phase is dried and concentrated. The 2-amino-6-n-propylamino-4,5,6,7-tetrahydro-benzthiazole dihydrochloride crystallizes out when ethereal hydrochloric acid is added. Yield: 42%. Melting point: 286°-288°C. |
| Therapeutic Function | Antiparkinsonian, Antipsychotic |
| Biological Activity | pramipexole is a dopamine agonist of the non-ergoline class indicated for treating parkinson's disease (pd) and restless legs syndrome (rls).pramipexole also possesses low/insignificant affinity (500-10,000 nm) for the 5-ht1a, 5-ht1b, 5-ht1d, and α2-adren |
Product picture

Packing &shipping&Payment
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $9.00/1KG |
Wuhan Boyuan Import & Export Co., LTD
|
2023-07-24 | ||
| $150.00/1kg |
VIP1Y
|
Henan Bao Enluo International TradeCo.,LTD
|
2023-05-12 | |
| $400.00/1KG |
Weijer International Trade (Hebei) Co., Ltd
|
2023-03-09 | ||
| $10.00/1kg |
Hebei Anda Technology Co., LTD
|
2022-09-26 | ||
| $9.90/1KG |
VIP6Y
|
Hebei Guanlang Biotechnology Co., Ltd.
|
2021-09-28 | |
| $1.10/1g |
VIP4Y
|
Dideu Industries Group Limited
|
2021-07-28 | |
| $15.00/1KG |
Zhuozhou Wenxi import and Export Co., Ltd
|
2021-06-26 | ||
| $126.00/25g |
Baoji Guokang Healthchem co.,ltd
|
2021-06-03 | ||
| $1.00/1KG |
VIP6Y
|
Career Henan Chemical Co
|
2018-08-20 | |
| $50.00/1KG |
Zhuozhou Wenxi import and Export Co., Ltd
|
2020-09-09 |
- Since: 2017-12-08
- Address: Building A, Enjoy city, Zhongshan East Road, Shijiazhuang city, Hebei province
13288715578
sales@hbmojin.com











 China
China