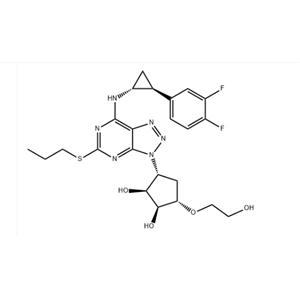
TICAGRELOR NEW
| Price | Get Latest Price | ||
| Package | 1kg | 10kg | 100kg |
| Min. Order: | 1kg |
| Supply Ability: | 20tons |
| Update Time: | 2023-08-04 |
Product Details
| Product Name: TICAGRELOR | CAS No.: 274693-27-5 |
| Min. Order: 1kg | Purity: 99% |
| Supply Ability: 20tons | Release date: 2023/08/04 |
Properties of Ticagrelor Melting point 138-140 ° C Boiling point 777.6 ± 70.0 ° C (Predicted) Density 1.67 Storage conditions Keep indarkplace, Inertatmosphere, Storeinfreezer, under -20 ° C Solubility Methanol (Slightly) Acid dissociation constant (pKa) 13.26 ± 0.70 (Predicted) Form Solid Color WhitetoOff White Stability Ticagrelor Uses and synthesis Antiplatelet agglutination drug Ticagrelor is a new type of antiplatelet agglutination drug, Developed by AstraZeneca, it is the first reversible binding oral P2Y12 adenosine diphosphate receptor antagonist in the world. It can reversibly act on the purinoceptor2 (P2) subtype P2Y12 on vascular smooth muscle cells (VSMC) without metabolic activation, and has obvious inhibitory effect on platelet aggregation caused by Adenosine diphosphate (ADP), It can effectively improve the symptoms of patients with acute coronary heart disease. Different from thienopyridine drugs, Ticagrelor is a reversible inhibitor of P2Y12 receptor, so it is particularly suitable for patients who need to undergo surgery after anticoagulation treatment in advance. AstraZeneca began to develop Ticagrelor in 1999, and the European Heart Association (ESC) first announced the results of the Phase III trial of Ticagrelor at its 2009 annual meeting, describing and comparing its efficacy in patients with acute coronary syndrome (ACS) in detail. In November 2009, AstraZeneca submitted a new drug marketing application for ticagrel to the European Union and the US FDA respectively. In December 2010, it was approved by the European Union for the prevention of Atherosclerosis thrombosis in adult patients with acute coronary syndrome (ACS). On December 17, 2010, the US Food and Drug Administration (FDA) again decided to postpone the approval of AstraZeneca's new antiplatelet drug Ticagrelor. FDA sent a letter to the company requesting to provide additional analysis of PLATO study on platelet inhibition and patient prognosis. In January 2011, it was officially sold in all member states of the European Union as Brilique, with a specification of 90mg/tablet and a 60 tablet package. On July 20, 2011, AstraZeneca announced that FDA had approved Ticagrelor to reduce cardiovascular death and heart attack in patients with acute coronary syndrome (ACS). So far, Ticagrelor has been approved to be listed in 41 countries and included in the medical compensation scope of 7 countries (such as the United Kingdom).
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $40.00/10mg |
VIP6Y
|
TargetMol Chemicals Inc.
|
2025-11-10 | |
| $40.00/10mg |
VIP4Y
|
TargetMol Chemicals Inc.
|
2025-11-10 | |
| $/ |
VIP5Y
|
RongNa Biotechnology Co.,Ltd
|
2025-04-29 | |
| $40.00/10mg |
VIP2Y
|
TargetMol Chemicals Inc.
|
2025-04-29 | |
| $0.00/1g |
VIP2Y
|
BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD.
|
2024-12-25 | |
| $100.00/25KG |
VIP7Y
|
Hebei Chuanghai Biotechnology Co., Ltd
|
2024-11-06 | |
| $0.00/1kg |
VIP2Y
|
Shaanxi TNJONE Pharmaceutical Co., Ltd
|
2024-05-21 | |
| $0.00/25kg |
PUSHAN INDUSTRIAL (SHAANXI) CO.,LTD
|
2024-05-15 | ||
| $10.00/1kg |
VIP2Y
|
Henan Fengda Chemical Co., Ltd
|
2024-04-29 | |
| $100.00/1kg |
Henan Bao Enluo International TradeCo.,LTD
|
2023-08-22 |


![3-[(3-CholaMidopropyl)diMethylaMMonio]-1-propanesulfonate hydrate](https://img.chemicalbook.com/ProductImageEN/2023-08/Large/7458f01b-a99c-40d7-b571-399e571fbd6d.png)

 China
China