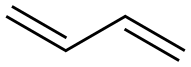1,3-Butadiene: Production, applications and environmental hazards
May 23,2023
General description
1,3-Butadiene is a colorless gas that is easily condensed to a liquid. It is important industrially as a monomer in the production of synthetic rubber. The molecule can be viewed as the union of two vinyl groups. It is the simplest conjugated diene. Although 1,3-Butadiene breaks down quickly in the atmosphere, it is nevertheless found in ambient air in urban and suburban areas as a consequence of its constant emission from motor vehicles. In 1863, the French chemist E. Caventou isolated butadiene from the pyrolysis of amyl alcohol.[1] This hydrocarbon was identified as 1,3-Butadiene in 1886, after Henry Edward Armstrong isolated it from among the pyrolysis products of petroleum. In 1910, the Russian chemist Sergei Lebedev polymerized 1,3-Butadiene and obtained a material with rubber-like properties. This polymer was, however, found to be too soft to replace natural rubber in many applications, notably automobile tires. Its appearance is as follows:

Figure 1 Appearance of 1,3-Butadiene.
Production
In the United States, western Europe, and Japan, 1,3-Butadiene is produced as a byproduct of the steam cracking process used to produce ethylene and other alkenes. When mixed with steam and briefly heated to very high temperatures (often over 900 °C), aliphatic hydrocarbons give up hydrogen to produce a complex mixture of unsaturated hydrocarbons, including 1,3-Butadiene. The quantity of 1,3-Butadiene produced depends on the hydrocarbons used as feed. Light feeds, such as ethane, give primarily ethylene when cracked, but heavier feeds favor the formation of heavier olefins, 1,3-Butadiene, and aromatic hydrocarbons. 1,3-Butadiene is typically isolated from the other four-carbon hydrocarbons produced in steam cracking by extractive distillation using a polar aprotic solvent such as acetonitrile, N-methyl-2-pyrrolidone, furfural, or dimethylformamide, from which it is then stripped by distillation.[2]
Applications
Most 1,3-Butadiene is polymerized to produce synthetic rubber. Polybutadiene itself is a very soft, almost liquid material of commercial interest. The copolymers prepared from 1,3-Butadiene and styrene and/or acrylonitrile, such as acrylonitrile butadiene styrene (ABS), nitrile-butadiene (NBR) and styrene-butadiene (SBR) are tough and/or elastic. SBR is the material most commonly used for the production of automobile tires.[3] Smaller amounts of 1,3-Butadiene are used to make the nylon intermediate, adiponitrile, by the addition of a molecule of hydrogen cyanide to each of the double bonds in a process called hydrocyanation developed by DuPont. Other synthetic rubber materials such as chloroprene, and the solvent sulfolane are also manufactured from 1,3-Butadiene. 1,3-Butadiene is used in the industrial production of 4-vinylcyclohexene via a Diels Alder dimerization reaction. Vinylcyclohexene is a common impurity found in 1,3-Butadiene upon storage due to dimerization. Cyclooctadiene and cyclododecatriene are produced via nickel- or titanium-catalyzed dimerization and trimerization reactions, respectively. 1,3-Butadiene is also useful in the synthesis of cycloalkanes and cycloalkenes, as it reacts with double and triple carbon-carbon bonds through the Diels-Alder reaction.
Environmental hazards
1,3-Butadiene is of low acute toxicity. LC50 is 12.5-11.5 vol% for inhalation by rats and mice.[3] Long-term exposure has been associated with cardiovascular disease, there is a consistent association with leukemia, as well as a significant association with other cancers. 1,3-Butadiene has been designated a Group 1 carcinogen ('carcinogenic to humans') by IARC,[4] and has also been listed as a carcinogen by the Agency for Toxic Substances Disease Registry and the US EPA. The American Conference of Governmental Industrial Hygienists (ACGIH) lists the chemical as a suspected carcinogen. The Natural Resource Defense Council (NRDC) lists some disease clusters that are suspected to be associated with this chemical. Some researchers have concluded it is the most potent carcinogen in cigarette smoke, twice as potent as the runner up acrylonitrile[5] 1,3-Butadiene is recognized as a Highly Reactive Volatile Organic Compound (HRVOC) for its potential to readily form ozone, and as such, emissions of the chemical are highly regulated by TCEQ in parts of the Houston-Brazoria-Galveston Ozone Non-Attainment Area.
References
[1]Caventou, E. (1863). "Ueber eine mit dem zweifach-gebromten Brombutylen isomere Verbindung und über die bromhaltigen Derivate des Brombutylens". Justus Liebigs Annalen der Chemie. 127: 93–97.
[2]Sun, H.P. Wristers, J.P. (1992). Butadiene. In J.I. Kroschwitz (Ed.), Encyclopedia of Chemical Technology, 4th ed., vol. 4, pp. 663–690. New York: John Wiley & Sons.
[3]J. Grub, E. L?ser (2012). "Butadiene". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a04_431.pub2.
[4]Grosse, Yann; Baan, Robert; Straif, Kurt; Secretan, Béatrice; El Ghissassi, Fatiha; Bouvard, Véronique; Altieri, Andrea; Cogliano, Vincent (2008). "Carcinogenicity of 1,3-butadiene, ethylene oxide, vinyl chloride, vinyl fluoride, and vinyl bromide". The Lancet Oncology. 8 (8): 679–680. doi:10.1016/S1470-2045(07)70235-8. ISSN 1470-2045. PMID 17726789.
[5]Fowles, J; Dybing, E (4 September 2003). "Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke". Institute of Environmental Science and Research. 12 (4): 424–430. doi:10.1136/tc.12.4.424. PMC 1747794. PMID 14660781.
- Related articles
- Related Qustion
- 1,3-Butadiene: Overview, Applications in Synthetic Rubber and Biosynthesis May 13, 2024
Recent advancements allow biosynthesis of 1,3-butadiene from glucose in microorganisms, pivotal for synthetic rubber production, enhancing sustainability.
- 1,3-Butadiene: pathogenicity and synthesis Dec 18, 2023
Occupational exposure to 1,3-Butadiene poses cancer and cardiovascular risks, with no safe exposure level, and 1,3-Butadiene is synthesized using high-temperature reactions.
- Butadiene:Mechanism of Toxicity Nov 30, 2021
Butadiene is produced during the combustion of organic matter. Significant amounts of butadiene are released to the environment from both natural and anthropogenic sources such as forest fires, gasoline and diesel engine exhaust, and wood s
Triadimefon is a systemic fungicide in the triazole family of chemicals, which are also called triazole or imidazole-containing fungicides.....
May 22,2023Pesticide IntermediatesAstaxanthin is one of the most sought-after antioxidant supplements right now – and with good reason. It’s not only an antioxidant powerhouse but also jam-packed with anti-fatigue and anti-inflammator....
May 23,2023Food Additives1,3-Butadiene
106-99-0You may like
- Biethylene
-

- $1.00 / 1KG
- 2025-12-11
- CAS:106-99-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- 1,3-Butadiene
-

- $50.00 / 1kg
- 2025-09-26
- CAS:106-99-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20Tons
- 1,3-Butadiene
-
- $101.00 / 1KG
- 2025-09-25
- CAS:106-99-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available