5-Bromo-5-nitro-1,3-dioxane: Analytical Method, Skin Penetration & Safety
Dec 8,2025
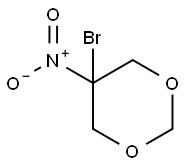
5-bromo-5-nitro-1,3-dioxane is an organobromide that is a nitrobromo derivative of dioxane. It is used as a stabilizer, surfacant, bactericide, and a preservative in immunology and cosmetics. It is corrosive to metals. 5-bromo-5-nitro-1,3-dioxane has been used in cosmetics since the mid-1970s as preservative for shampoos and foam baths.

The determination of 5-bromo-5-nitro-1,3-dioxane in rinse-off cosmetics
Cosmetics are important consumer products with an essential role in everyone's life: apart from traditional cosmetic products, such as make-up and perfumes, it also includes products for personal hygiene, for example tooth-care products, shampoos and soaps. The addition of preservatives is absolutely essential to ensure that cosmetics are safe to use for a long time, since these compounds protect formulations from contamination by microorganisms such as bacteria and yeasts. 5-Bromo-5-nitro-1,3-dioxane (bronidox) is a bromine-containing preservative often used in cosmetics and pharmaceutical preparations including shampoos, body cleansers and facial exfoliants. Bronidox has recently attracted attention as potential allergen and as nitrosating agent. According to the current European legislation, this preservative is restricted to rinse-off cosmetics with a maximum authorized concentration (MAC) of 0.1% and with the requirement of avoiding the formation of nitrosamines. 5-Bromo-5-nitro-1,3-dioxane is even not permitted in other more preventive legislations, as the Japanese. Even though solid-phase microextraction has been previously applied to the analysis of other kind of preservatives (parabens) in cosmetic samples, it is important to highlight that this is the first time that 5-Bromo-5-nitro-1,3-dioxane has been extracted by SPME, which is considered as a valuable alternative analytical technique to more traditional procedures, reducing the laboratory-generated waste and time for sample preparation.[1]
An experimental design was set up to evaluate the simultaneous effect of the main parameters affecting the SPME extraction of 5-Bromo-5-nitro-1,3-dioxane from rinse-off cosmetics. The studied variables were: type of fiber coating (A), extraction temperature (B), sampling mode (C) and addition of sodium chloride (D). First of all, preliminary experiments using PDMS, PDMS/DVB, PA, CAR/PDMS and DVB/CAR/PDMS coatings were performed in order to select the levels of this factor to be included in the above-mentioned experimental design. This paper describes the optimization, validation and application of a novel SPME–GC-μECD method for the determination of 5-Bromo-5-nitro-1,3-dioxane in rinse-off cosmetics. An experimental design allowed estimating the effect of several SPME variables simultaneously. The proposed methodology has the advantages of short duration, simplicity and absence of organic solvents. Furthermore, the obtained results indicated that this method is capable of providing sensitive, reproducible and accurate data for the analyses of several types of formulations such as shampoos, gels or facial exfoliants. Twenty-two cosmetic products were analyzed employing the recommended procedure and it was demonstrated that all of them were labeled correctly with respect to the presence of 5-Bromo-5-nitro-1,3-dioxane.
In vitro skin penetration of 5-bromo-5-nitro-1,3-dioxane
The use of bronidox (5-Bromo-5-nitro-1,3-dioxane), bronopol (2-Bromo-2-nitropropane-1,3-diol) and dimethyloldimethyl hydantoin (DMDM hydantoin) preservatives authorized in current regulations for use in cosmetic products has increased to avoid the use of parabens since although these are not prohibited, there are indications of their possible danger). As regards bronopol and 5-bromo-5-nitro-1,3-dioxane (and formaldehyde before being prohibited), the European legislation has established maximal allowed concentrations as 0.1% (w/w) while DMDM hydantoin maximal concentration allowed is 0.6% (w/w). There are no studies related to skin permeability of the preservers above mentioned. Therefore, the aims of the present work were (i) to evaluate the skin permeation of bronopol, 5-bromo-5-nitro-1,3-dioxane, and formaldehyde, and formaldehyde released from bronopol and DMDM hydantoin in vitro through porcine ear skin under infinite dose conditions and (ii) to investigate the effect of sample matrix on the preservatives skin permeation and formaldehyde release from these donors.[2]
The degree of absorption of 5-bromo-5-nitro-1,3-dioxane and bronopol when administered on the skin depends on the formulation containing both preservatives. O/W emulsions are the assayed systems that least promote transdermal absorption of 5-bromo-5-nitro-1,3-dioxane while the transdermal absorption of bronopol was lower from a hydrogel. Both preservatives reach maximal transdermal absorption when formulated in an aqueous solution. Moreover, the transdermal absorption of formaldehyde when bronopol is used also depends on the formulation components, being the aqueous solution the worst system because more formaldehyde is absorbed through skin. Taking into account the concentrations authorized by the European regulations and once calculated for each preservative the percentages that could reach the bloodstream, it can be concluded that the use of bronopol and 5-bromo-5-nitro-1,3-dioxane under the conditions required by the current regulations are safe.
References
[1]Fernandez-Alvarez, Maria et al. “Development of a solid-phase microextraction gas chromatography with microelectron-capture detection method for the determination of 5-bromo-5-nitro-1,3-dioxane in rinse-off cosmetics.” Journal of chromatography. A vol. 1217,43 (2010): 6634-9. doi:10.1016/j.chroma.2010.04.027
[2]López-Sánchez, Lucía et al. “In vitro skin penetration of bronidox, bronopol and formaldehyde from cosmetics.” Regulatory toxicology and pharmacology : RTP vol. 122 (2021): 104888. doi:10.1016/j.yrtph.2021.104888
- Related articles
- Related Qustion
Lithium deuteride exhibits distinct phonon/thermoelastic isotope effects, and serves in nuclear weapons/energy storage.....
Dec 8,2025API(R)-(+)-Tetrahydro-2-furoic acid is efficiently synthesized via enzyme engineering, and forms H-complexes via carbonyl/heterocycle oxygen.....
Dec 8,2025Chemical Materials5-Bromo-5-nitro-1,3-dioxane
30007-47-7You may like
5-Bromo-5-nitro-1,3-dioxane manufacturers
- 5-Bromo-5-nitro-1,3-dioxane
-

- $0.00 / 1G/KG
- 2025-12-11
- CAS:30007-47-7
- Min. Order: 1G/KG
- Purity: 99%
- Supply Ability: 100MT
- 5-Bromo-5-nitro-1,3-dioxane
-

- $0.00 / 25Kg/Drum
- 2025-12-11
- CAS:30007-47-7
- Min. Order: 1KG
- Purity: 99%min
- Supply Ability: 10 tons
- 5-Bromo-5-nitro-1,3-dioxane
-
- $66.00 / 500mg
- 2025-12-05
- CAS:30007-47-7
- Min. Order:
- Purity: 99.87%
- Supply Ability: 10g