5-Bromo-7-azaindole:Synthesis,XRD analysis and solubility under different condition
May 19,2025
Introduction
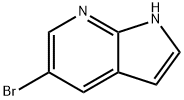
7-Azaindole and its derivatives exhibit significant biological activities and the framework has contributed to the development of new therapeutic agents. For example, natural products, meriolin 1 and meriolin 3 showed improved potency, and several FDA-approved drugs such as vemurafenib are used for the treatment of metastatic melanoma. Pexidartinib is approved for the treatment of giant cell tumours associated with severe morbidity. Moreover, 7-azadindole derivative NVP-QAV680 is known for the treatment of allergies and Zn-azaindole complex has a bright blue emitter property.[1] 5-Bromo-7-azaindole (C7H5BrN2; molar mass: 197.03 g·mol-1 ; CAS: 183208-35-7; Fig. 1) is a yellow compound similar in structure to indole and purine. It is an intermediate in laboratory organic synthesis and chemical pharmaceutical research and development. It is an intermediate in novel antitumor drugs, active drug vemurafenib and ABT199[2].5-bromo-7-azaindole can be combined with CAC, CAN and CAO through various coupling reactions, thus completing the design and synthesis of new drug molecules[3].

Synthesis process of 5-Bromo-7-azaindole
The 5-Bromo-7-azaindole synthesis process comprises the steps that 7-azaindole, raney nickel and ethyl alcohol are stirred and hydrogen is led for reaction; a reactant is filtered, a filter cake is washed by using ethyl alcohol, and filtrates are mixed and concentrated to obtain a crude 7-azaindole product; the crude product is mixed with p-toluenesulfonic acid and dichloromethane, and bromine is dropwise added and stirred; reaction liquid is washed by using sodium thiosulfate, an organic phase is dried by using anhydrous sodium sulfate, and concentration is performed to obtain a 5-Bromo-7-azaindole product; the product is dissolved in methylbenzene, and manganese dioxide is added to perform heating reflux reaction; the reaction liquid is filtered, the filter cake is washed by using dichloromethane, the organic phases are mixed, dried and concentrated to obtain a crude 5-Bromo-7-azaindole product, and crystallization is performed.[4]
The crystal structure, vibrational spectroscopic studies [2]
5-Bromo-7-azaindole (5Br7AI) may act as the carrier ligand for platinum(II), in new anticancer agent, cis-[PtCl2(5Br7AI)2]. The XRD analysis of 5-bromo-7-azaindole (5Br7AI) was reported recently. However, these authors used some restrictions in the determination of the structure (for example, the N-H bond length was fixed at 0.88 Å and also the H-N-C bond angles were fixed at identical values). In Barbara’s single crystal X-ray analysis, the coordinates and isotropic displacement parameters of all hydrogen atoms have been refined freely. Thus, the geometrical parameters of the dual N–H···N hydrogen bonds, determined in this work, are more accurate than those reported earlier. The crystal and molecular structure of the title molecule has been reinvestigated by a single crystal X-ray diffraction. The FT-IR and FT-Raman spectra of 5-bromo-7-azaindole and its N-deuterated derivative have been recorded. The molecular structures of monomer and dimer, natural charges on atoms and theoretical vibrational spectra have been studied by density functional B3LYP method using 6-311++G(d,p) basis set. The results have shown that in the crystal, a pair of 5-bromo-7-azaindole molecules forms a centrosymmetric dimer linked by the moderately strong, dual and nearly linear N–H···N hydrogen bonds between the pyrrole and pyridine rings. The optimized geometry of the (5Br7AI)2 dimer and the calculated spectra show very good agreement with the experiment. Detailed vibrational assignments for all the species have been made on the basis of the calculated potential energy distributions (PEDs). It is concluded that a complicated spectral features of the NH (ND) stretching absorption bands are due to multiple Fermi resonances, and they are characteristic for the doubly hydrogen bonded N–H···N dimers of 7-azaindole and its halogeno derivatives.
Analysis of solubility of 5-bromo-7-azaindole
This research adopted the static balance method to study the solubility of 5-bromo-7-azaindole impurity indole in ten kinds of pure solvents (methanol, ethyl acetate, n-butyl alcohol, n-propanol, isopropanol, dichloromethane(DCM), acetonitrile, methyl acetate, ethanol and n-hexane) and four mixed solvents (n-hexane + methyl acetate, n-hexane +ethyl acetate, n-hexane + ethanol and n-hexane + dichlorome thane) at atmospheric pressure. The research experimental temperatures ranged from 278.15 to 323.15 K. The aim of this paper is to study the solubility of 5-bromo-7-azaindole in different solvent systems, and to provide reference for selecting suitable solvents in crystallization process. The modified Apelblatand and λh models were used to fit the solubility data of pure solvents. RMSDmax were 0.080 % and 0.29 %, respectively. Meanwhile, the CNIBS/R-K model and Jouyban–Acree model were in good agreement with the solubility data of binary solvent mixtures. RMSDmax were 0.043% and 0.031%, respectively. The KAT-SER model was used to investigate the interaction of 5-bromo-7- azaindole in pure solvent and the effect of polarity on the solubility of hydrogen bond in solute solvent system. The results showed that the hydrogen bond donor capacity accounted for 33.26 % of the total solvent effect.[3]
References
[1] Pavithra E, Kannadasan S, Shanmugam P. Synthesis of 5-aryl-3,3'-bis-indolyl and bis-7-aza-indolyl methanone derivatives from 5-bromo-7-azaindoles via sequential methylenation using microwave irradiation, CAN oxidation, and Suzuki coupling reactions. RSC Adv. 2022;12(47):30712-30721. Published 2022 Oct 27. doi:10.1039/d2ra05849a
[2] Morzyk-Ociepa B , Dysz K , Turowska-Tyrk I ,et al.Reinvestigation of the crystal structure, vibrational spectroscopic studies and DFT calculations of 5-bromo-7-azaindole with dual N–HN hydrogen bonds in dimers[J].Journal of Molecular Structure, 2015:91-100.DOI:10.1016/j.molstruc.2015.08.003.
[3] Bao S , Sun N , Sun W ,et al.Determination and Analysis of solubility of 5-bromo-7-azaindole in pure and mixed solvent systems at Different Temperatures (T = 278.15-323.15 K)[J].Journal of Molecular Liquids, 2022.DOI:10.1016/j.molliq.2022.120476.
[4] Xia Qq,et al. 5-Bromo-7-azaindole synthesis process.2016[2025-04-28].
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringThis article mainly summarizes the pharmacokinetic studies of maropitant citrate in different species under different administration methods.....
May 20,2025Chemical Materials5-Bromo-7-azaindole
183208-35-7You may like
5-Bromo-7-azaindole manufacturers
- 5-Bromo-7-azaindole
-

- $0.00 / 1kg
- 2025-12-13
- CAS:183208-35-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20MT
- 5-Bromo-7-azaindole
-

- $0.00 / 100KG
- 2025-12-12
- CAS:183208-35-7
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20mt
- 5-bromo-7-azaindole
-

- $0.00 / 25KG
- 2025-12-12
- CAS:183208-35-7
- Min. Order: 1KG
- Purity: ≥99.0%
- Supply Ability: Ton