Adverse effect of Sugammadex
Dec 8,2021
General description
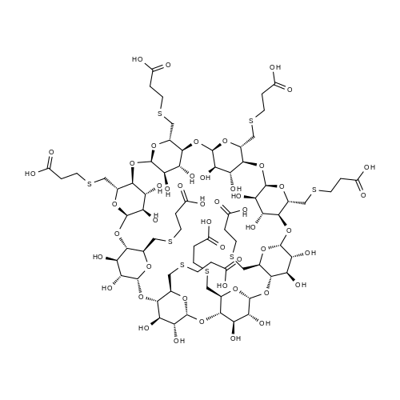
Sugammadex is a biologically inert, selective relaxant binding agent (SRBA) composed of a modified, anionic gamma cyclodextrin derivative containing a hydrophilic exterior and a hydrophobic core, with neuromuscular blocking drug (NMBD) reversal activity. Upon administration, the negatively charged carboxyl-thio-ether groups of sugammadex selectively and reversibly bind to the positively charged quaternary nitrogen of a steroidal NMBD, such as rocuronium and vecuronium, which was administered at an earlier time for anesthetic purposes. The encapsulation of the NMBD by sugammadex blocks its ability to bind to nicotinic receptors in the neuromuscular junction and thereby reverses the NMBD-induced neuromuscular blockade.[1]
Sugammadex is an octasaccharide derivative that is gamma-cyclodextrin in which all eight primary hydroxy groups are replaced by 2-(carboxyethyl)sulfanyl groups. Used (as the octasodium salt) for reversal of neuromuscular blockade induced by rocuronium and vecuronium in adults undergoing surgery. It has a role as a neuromuscular agent. It is an octasaccharide derivative and an organic sulfide. It derives from a gamma-cyclodextrin. It is a conjugate acid of a sugammadex(8-).
Application and Pharmacology
Sugammadex is a reversal agent that was engineered to reverse the effects of aminosteroid muscle relaxants. It is a modified gamma-cyclodextrin, i.e. a large glucose molecule bound in a ring-like structure. Sugammadex, when injected intravenously, creates a concentration gradient favoring the movement of aminosteroid muscle relaxants from the neuromuscular junction back into the plasma, and then encapsulates the aminosteroid muscle relaxants within its inner structure by forming tight water-soluble complexes.
Case reports discuss the use of sugammadex in pediatric patients with neuromuscular diseases. Although sugammadex is typically used in the operating room for reversing neuro-muscular blockade for surgical procedures, there is a small but importantrole for sugammadex use in the emergency department. In cases where rapid neurological examination is required after neuromuscular blockage with rocuronium or vecuronium, sugammadex can assist in facilitating a timely comprehensive neurological examination where pharmacologic or surgical management may depend on examination findings such as in the case of cerebral vascular accident, status epilepticus, or traumatic brain injury. Some clinicians have advocated for the use of sugammadex in the cannot intubate, cannot ventilate scenario. However, caution should be exercised in this situation as reversal of paralysis can take up to 22 minutes to occur.
Neuromuscular blockade is induced with the administration of depolarizing and nondepolarizing NMBA. Succinylcholine, an ultrashort depolarizing NMBA, initially activates skeletal muscles, which is then followed by neuromuscular blockade and paralysis.6Nondepolarizing NMBAs, on the other hand, competitively inhibit ACh by binding to the postsynaptic nicotinic ACh receptors without activating the receptor. Succinylcholine-induced paralysis cannot be reversed by sugammadex.[1,2]
Synthesis
The industrial production of Shugeng glucose sodium is difficult, and there are many difficulties in the analysis of impurities. In order to find a method for the synthesis of Shugeng glucose sodium with high purity, high yield, environmental protection, easy removal of by-products and mild reaction conditions, the production method suitable for industrial Shugeng glucose sodium and its intermediates has been found through in-depth research.
Reagents and instruments:γ- Cyclodextrin,; Bromine, triphenylphosphine, 3-mercaptopropionic acid, sodium hydroxide and sodium hydride are all domestic analytical pure; N. N-dimethylformamide (DMF), dimethyl sulfoxide (dm-so), methanol and ethanol are imported chromatographic pure.
Scheme:Add 3-mercaptopropionic acid into DMSO, stir and drop NaOH solution. During the dropping process, control the temperature below 30 ℃, drop it, keep the temperature at 28 ~ 29 ℃, stir for 1h, drop DMSO solution of intermediate (intermediate ∶ 3-mercaptopropionic acid sodium = 1 ∶ 15, mass ratio, the same below), drop it, Heat up to 45 ℃ and stir for about 5h. Add ethanol, stir, filter, wash the filter cake with ethanol and dry to obtain Shugeng sodium gluconate coarse powder.[3,4]
Figure the systhesis route of Sugammadex sodium
γ- Cyclodextrin was chlorinated with oxalyl chloride. The method has the advantages of simple process, high yield, high purity and no pollution, and is suitable for industrial production. Using y-cyclodextrin as a raw material. we synthesized Sugammadex sodium crude powder through the steps of bromination and thioetherification. Moreover. we obtained Sugammadex sodium refined powder with the total yield of 71. 6% through purification. This process has the advantages of simple process, low energy consumption. non-pollution, simple operation - mild reaction conditions, and high yield, which has a good industrial production prospect.
History
Sugammadex is a selective relaxant binding agent indicated for reversal of neuromuscular blockade induced by rocuroium bromide and vecuronium bromide during surgery in adults. Rocuroium bromide and vecuronium bromide are neuromuscular blocking medications that cause temporary paralysis and are especially useful for general anesthesia, ventilation, or tracheal intubation that patients may require for surgery. Sugammadex provides a new treatment option to reverse the effects of those medications and possibly help patients recover sooner post-surgery. Sugammadex (brand name Bridion) is marketed by Merck Sharp and Dohme, and was approved by the United States FDA on December 15, 2015.Since its clinical introduction in 2008, sugammadex has demonstrated a high degree of safety and superior effectiveness compared to neostigmine when used to antagonize muscle relaxation produced by steroid nondepolarizing neuromuscular blockers. This includes its use in special populations, such as the elderly, children over 2 years old, and patients with renal, hepatic, or lung disease. In contrast, clinical evidence guiding its use during pregnancy, in women of childbearing potential, and in lactating women, is sparse. [5]
Toxicity and adverse effect
There are ongoing clinical trials investigating its use in the pediatric population. Before approval in use in the United States, several adverse effects were noted to occur in patients receiving sugammadex in clinical trials including prolonged QT interval, bradycardia, hypersensitivity reactions, and prolongation of coagulation parameters. Additional investigations further elucidated the risks of these adverse events. Sugammadex is approved for use in children older than 2 years in other countries in Europe and Asia. Investigations suggest that the efficacy, safety, and pharmacokinetic profile is similar in children when compared with adults. Published pediatric data favor the use of sugammadex in children older than 2 years, but there are some data in young children younger than 2 years. [1] Recurrence of neuromuscular blockade can occur if providers choose to use lower than the recommended doses of sugammadex, despite initial reversal of neuromuscular blockade.17,18Neuromuscular blockade may also recur if a drug is given, which displaces the steroidal NMBA from sugammadex. Manufacturers single out toremifene, a selective estrogen receptor modulator, as having a high binding affinity for sugammadex, rendering it less effective at binding rocuronium or vecuronium. In addition, drugs that potentiate neuromuscular blockade can cause recurrence of paralysis and need for ventilatory support. The current paucity of evidence regarding sugammadex in women of childbearing potential warrants careful consideration when selecting NMB drugs and reversal agents for use in these women and suggests areas for further research designed to close evidence gaps that limit potential benefits of sugammadex in this population.
Reference
1.Hawkins J., Khanna S. & Argalious M., "Sugammadex for Reversal of Neuromuscular Blockade: Uses and Limitations," Current pharmaceutical design, Vol.25, No.19(2019), p.2140.
2.Richardson M. G. & Raymond B. L., "Sugammadex Administration in Pregnant Women and in Women of Reproductive Potential," Anesthesia & Analgesia, Vol.130, No.6(2020), pp.1628-1637.
3.Yang Jialiang, Roger, Xie Yongmei: Study on the synthetic process of Shugeng glucose sodium, chemical engineering and equipment, 2016, issue 6, pages 4-6。
4.Optimization in Synthetic Process of Sugammadex Sodium," Chemistry & Bioengineering, Vol.36, No.7(2019), pp.54-58.]
5. "Sugammadex,".
- Related articles
- Related Qustion
- Sugammadex: A revolutionary drug in neuromuscular pharmacology Nov 1, 2019
Sugammadex (ORG 25969) is a unique neuromuscular reversal drug; a novel cyclodextrin, the first in a new class of selective relaxant binding agents, which reverse neuromuscular blockade (NMB) with the aminosteroid non-depolarizing muscle re
- What is sugammadex Nov 1, 2019
Sugammadex can make hormonal birth control less effective. For at least 7 days after you receive sugammadex, use a non-hormonal back-up birth control (condom or diaphragm with spermicide) to prevent pregnancy. Hormonal contraception (birth
Gestodene is a steroid. It has a role as an estrogen.Gestodene, one of three new gonane progestins, is the most potent on a per weight basis in regard to progestational effects and has little or no es....
Dec 8,2021Drugsβ-Nicotinamide adenine dinucleotide (β-NAD) has been used for the preparation of deacetylated tubulin. It has also been used for UDP-glucose-6-hydrogenase (UGDH) enzyme activity assay of orital fibroblast cell lysates.....
Dec 8,2021Vitamins and Minerals medicinesSUGAMMADEX
343306-71-8You may like
- Sugammadex
-

- $0.00 / 1Kg/Bag
- 2025-12-18
- CAS:343306-71-8
- Min. Order: 1KG
- Purity: 99%min HPLC
- Supply Ability: 100kgs
- Sugammadex
-

- $0.00 / 1kg
- 2025-12-16
- CAS:343306-71-8
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 800kg
- Sugammadex
-

- $0.00 / 25KG
- 2025-12-01
- CAS:343306-71-8
- Min. Order: 1KG
- Purity: 98.0%
- Supply Ability: 10000KGS