Application of Fmoc-N-Me-Arg(pbf)-OH
Dec 9,2022
Physicochemical property
Fmoc-N-Me-Arg(pbf)-OH has a boiling point is predicted to be 817.5±75.0 °C and its density is predicted to be 1.34±0.1 g/cm3. It needs to be stored in a sealed dry, -20℃ refrigerator.
Application
Used to synthesize Neurotensin analogs
Since neurotensin (NT) receptors of subtype-1 (NTS1) are expressed by different types of malignant tumors, such as pancreatic adenocarcinoma, colorectal and prostate carcinoma, they represent an interesting target for tumor imaging by positron emission tomog. (PET) and endoradiotherapy. Fmoc-N-Me-Arg(pbf)-OH was used to synthesize Neurotensin analogs. Previously reported neurotensin-derived NTS1 ligands for PET were radiolabeled by modification and prelongation of the N-terminus of NT peptide analogs. In this study, we demonstrate that modifying Arg8 or Arg9 by Nω-carbamoylation and subsequent fluoroglycosylation provides a suitable approach for the development of NT analogs as PET imaging agents. The Nω-carbamoylated and fluoroglycosylated NT analogs retained high NTS1 affinity in the one-digit nanomolar range as well as high metabolic stability in vitro. In vivo, the radioligand [18F]Nα-methyl-(Nω-{N-[4-(3-{1-[6-deoxy-6-fluoro-β-D-glucopyranosyl]-1H-1,2,3-triazol-4 -yl}propanoyl)aminobutyl]aminocarbonyl})Arg-Arg-Pro-Tyr-2-tert-butyl-Gly-Leu-OH tris(hydrotrifluoroacetate) demonstrated favorable biokinetics in HT-29 tumor-bearing mice with high tumor uptake and high retention, predominantly renal clearance, and fast wash-out from blood and other non-target tissues. Therefore, [18F]Nα-methyl-(Nω-{N-[4-(3-{1-[6-deoxy-6-fluoro-β-D-glucopyranosyl]-1H-1,2,3-triazol-4 -yl}propanoyl)aminobutyl]aminocarbonyl})Arg-Arg-Pro-Tyr-2-tert-butyl-Gly-Leu-OH tris(hydrotrifluoroacetate) has the potential to be used as mol. probe for the imaging of NTS1-expressing tumors by PET [1].
Methods for synthesizing β-homo-amino acids and disulfide-linked cyclic peptides
Nicotinamide N-methyltransferase (NNMT), which catalyzes the methylation of nicotinamide, is a cytosolic enzyme that has attracted much attention as a therapeutic target for a variety of diseases. However, despite the considerable interest in this target, reports of NNMT inhibitors have still been limited to date. Fmoc-N-Me-Arg(pbf)-OH is used to synthesize β-hom-amino acids and disulfide-linked cyclic peptides. In this work, utilizing in vitro translated macrocyclic peptide libraries, we identified peptide 1 as a novel class of NNMT inhibitors. Further exploration based on the X-ray cocrystal structures of the peptides with NNMT provided a dramatic improvement in inhibitory activity (peptide 23: IC50 = 0.15 nM). Furthermore, by balance of the peptides' lipophilicity and biol. activity, inhibitory activity against NNMT in cell-based assay was successfully achieved (peptide 26: cell-based IC50 = 770 nM). These findings illuminate the potential of cyclic peptides as a relatively new drug discovery modality even for intracellular targets [2].
Identification of a High-Affinity Tetrapeptide Agonist and a Hexapeptide Antagonist
Within the family of neuropeptide Y (NPY) receptors, the Y4 receptor (Y4R) is unique as it prefers pancreatic polypeptide (PP) over NPY and peptide YY (PYY). Fmoc-N-Me-Arg(pbf)-OH is used to synthesize Tetrapeptide Agonist and a Hexapeptide Antagonist. Today, low mol. wt. Y4R ligands are lacking, in particular antagonists. We synthesized a series of peptidic NPY Y4R ligands, derived from the hexapeptide acetyl-Arg-Tyr-Arg-Leu-Arg-Tyr-NH2 (1), reported to be a Y4R partial agonist with high affinity (pKi Y4R: 8.43). Peptide 1 was N-terminally extended as well as truncated, subjected to a D-amino acid scan, and Leu was replaced by different amino acids. Compds. were characterized by radioligand competition binding and functional studies (Cai2+ mobilization and β-arrestin 1/2 recruitment). N-terminal truncation of 1 resulted in a tetrapeptide (Arg-Leu-Arg-Tyr-NH2) being a Y4R partial agonist with retained Y4R affinity (pKi: 8.47). Remarkably, replacement of Leu in 1 and in derivs. of 1 by Trp turned Y4R agonism to antagonism, giving Y4R antagonists with pKi values ≤ 7.57 [3].
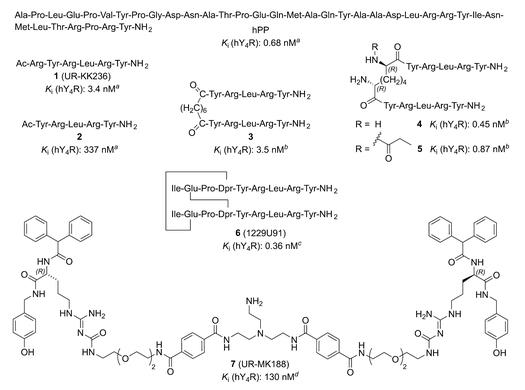
Figure 1. Structures and reported Y4R affinities of hPP, the Y4R (partial) agonists 1–6, and the nonpeptide Y4R antagonist 7.
Heterogeneous-Backbone Foldamer Mimics of Zinc Finger Tertiary Structure
A variety of oligomeric backbones with compns. deviating from biomacromols. can fold in defined ways. Termed "foldamers," these agents have diverse potential applications. A no. of protein-inspired secondary structures (e.g., helixes, sheets) have been produced from unnatural backbones, yet examples of tertiary folds combining several secondary structural elements in a single entity are rare. One promising strategy to address this challenge is the systematic backbone alteration of natural protein sequences, through which a subset of the side chains is displayed on an unnatural building block to generate a heterogeneous backbone. Here, Fmoc-N-Me-Arg(pbf)-OH is the heterogeneous backbone. A drawback to this approach is that substitution at more than one or two sites often comes at a significant energetic cost to fold stability. Here we report heterogeneous-backbone foldamers that mimic the zinc finger domain, a ubiquitous and biol. important metal-binding tertiary motif, and do so with a folded stability that is superior to the natural protein on which their design is based. A combination of UV-vis spectroscopy, isothermal titrn. calorimetry, and multidimensional NMR reveals that suitably designed oligomers with >20% modified backbones can form native-like tertiary folds with metal-binding environments identical to the prototype sequence (the third finger of specificity factor 1) and enhanced thermodn. stability. These results expand the scope of heterogeneous-backbone foldamer design to a new tertiary structure class and show that judiciously applied backbone modification can be accompanied by improvement to fold stability [4].
References
[1] Schindler L, Wohlfahrt K, Gluhacevic von Krüchten L, et al. Neurotensin analogs by fluoroglycosylation at Nω-carbamoylated arginines for PET imaging of NTS1-positive tumors[J]. Scientific reports, 2022, 12(1): 1-14.
[2] Hayashi K, Uehara S, Yamamoto S, et al. Macrocyclic peptides as a novel class of NNMT inhibitors: A SAR study aimed at inhibitory activity in the cell[J]. ACS Medicinal Chemistry Letters, 2021, 12(7): 1093-1101.
[3] Konieczny A, Braun D, Wifling D, et al. Oligopeptides as neuropeptide Y Y4 receptor ligands: identification of a high-affinity tetrapeptide agonist and a hexapeptide antagonist[J]. Journal of Medicinal Chemistry, 2020, 63(15): 8198-8215.
[4] George K L, Horne W S. Heterogeneous-backbone foldamer mimics of zinc finger tertiary structure[J]. Journal of the American Chemical Society, 2017, 139(23): 7931-7938.
- Related articles
- Related Qustion
- Fmoc-N-Me-Arg(pbf)-OH: Advancing Peptide Chemistry in Biological Applications Apr 25, 2024
Fmoc-N-Me-Arg(pbf)-OH facilitates peptide synthesis, bioorthogonal conjugation, and PET imaging, offering versatile applications in research and therapy.
The passage introduces the side effects and dosage of Ostarine.....
Dec 8,2022Biochemical EngineeringAPS-5 is mainly used as the core reagent of chemiluminescence substrates.....
Dec 9,2022Biochemical EngineeringFmoc-N-Me-Arg(pbf)-OH
913733-27-4You may like
- D-glucuronic acid: production methods and application
Oct 13, 2025
- Application research of Hexapeptide 9
Sep 4, 2025
- The study of Fmoc-L-Arg(Pbf)-OH
Aug 15, 2025
Fmoc-N-Me-Arg(pbf)-OH manufacturers
- Fmoc-N-Me-Arg(pbf)-OH
-

- $1.00 / 1KG
- 2019-07-06
- CAS:913733-27-4
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 1kg,5kg,100kg