Application research of R-(+)-lipoic acid
Jul 25,2025
Introduction
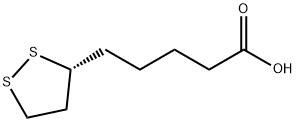
Alpha-Lipoic acid (ALA;Figure 1) is the chiral molecule of a drug having an asymmetric carbon atom existing in both R- and S-forms (Figure 2). A study has shown the approximately twofold higher bioavailability of the R-form of α-lipoic acid (R-LA), as compared to the S-form of α-lipoic acid (S-LA). In a dose/response curve model of R-(+)-lipoic acid and S-LA in the working rat heart during reoxygenation, the R-enantiomer was found to be more superior in the enhancement of the aortic flow. Moreover, in a model of sciatic nerve injury in hypertensive rats,R-(+)-lipoic acid was more active than S-LA in terms of analgesic and neuroprotective efficacy.This may be related to the better recognized dextrorotatory enantiomer by enzymes, as it was found that the maximum plasma concentration (Cmax) is approximately 40-50% higher with R-(+)-lipoic acid than with S-LA at the same dose. However, there are still gaps in the research on the effects of different enantiomers of ALA to alleviate the anti-inflammatory and antioxidant properties of the organism and oviduct in aged laying hens.[1] R-(+)-lipoic acid (also konwn as (R)-(+)-1,2-Dithiolane-3-pentanoic acid), a naturally occuring dithiol compound, is a potent antioxidant that can scavenge free radicals, chelate metal ions, and regenerate antioxidants. Currently, intravenous alpha-Lipoic acid is clinically used in the treatment of diabetic neuropathy. Besides, several studies have also reported the direct inhibitory effects of alpha-Lipoic acid on platelet aggregation which might through elevation of cyclic AMP formation, inhibition of intracellular Ca2+ and interaction with PKCα.[2]


R- is Superior to S-Form of alpha-Lipoic Acid
The development of single enantiomers with high efficiency and low toxic activity has become a hot spot for the development and application of drugs and active additives. The aim of the present study was to investigate the effectiveness of the application of α-lipoic acid with a different optical rotation to alleviate the inflammation response and oxidative stress induced by oxidized fish oil in laying hens. Sixty-four 124-week-old Peking Red laying hens were randomly allocated to four groups with eight replicates of two birds each. The normal group was fed basal diets supplemented with 1% fresh fish oil (FO), and the oxidative stress model group was constructed with diets supplemented with 1% oxidized fish oil (OFO). The two treatment groups were the S-form of the α-lipoic acid model with 1% oxidized fish oil (OFO+S-LA) and the R-(+)-lipoic acid model with 1% oxidized fish oil (OFO + R-LA) added at 100 mg/kg, respectively. Herein, these results were evaluated by the breeding performance, immunoglobulin, immune response, estrogen secretion, antioxidant factors of the serum and oviduct, and pathological observation of the uterus part of the oviduct. From the results, diets supplemented with oxidized fish oil can be relatively successful in constructing a model of inflammation and oxidative stress. The OFO group significantly increased the levels of the serum inflammatory factor (TNF-α, IL-1β, IL-6, and IFN-γ) and the oxidative factor MDA and decreased the activity of the antioxidant enzyme (T-AOC, T-SOD, GSH-Px, GSH, and CAT) in the oviduct. The addition of both S-LA and R-(+)-lipoic acid significantly reduced the levels of serum inflammatory factors (TNF-α, IL-1β, IL-6, and IFN-γ), increased the activity of antioxidant indexes (T-AOC, T-SOD, GSH-Px, GSH, and CAT), and decreased the MDA contents in the serum and oviduct. Meanwhile, the supplementation of S-LA and R-(+)-lipoic acid also mitigated the negative effects of the OFO on the immunoglobulins (IgA and IgM) and serum hormone levels (P and E2). In addition, it was worth noting that the R-(+)-lipoic acid was significantly more effective than the S-LA in some inflammatory (IL-1β) and antioxidant indices (T-SOD, GSH, and CAT). Above all, both S-LA and R-(+)-lipoic acid can alleviate the inflammation and oxidative damage caused by oxidative stress in aged laying hens, and R-(+)-lipoic acid is more effective than S-LA. Thus, these findings will provide basic data for the potential development of α-lipoic acid as a chiral dietary additive for laying hens.[1]
In addition,other researchers find that histone deacetylases HDAC1, HDAC2, HDAC3, HDAC6, HDAC8, and HDAC10 are molecular targets of the reduced form of lipoic acid and lipoamide. Importantly, only the naturally occurring R-(+)-lipoic acid inhibits HDACs at physiologically relevant concentrations and leads to hyperacetylation of HDAC substrates. The inhibition of HDACs by R-(+)-lipoic acid and lipoamide explain why both compounds prevent stress granule formation in cells and may also provide a molecular rationale for many other phenotypic effects elicited by lipoic acid.[3]
(R)-(+)-lipoic acid oral liquid formulation
R-(+)-lipoic acid is present in nature, and is found in animals, plants and from human body. In nature,this is the form which ALA demonstrates its effects. Despite its numerous potentials, oral administration of α-lipoic acid is characterised by pharmacokinetic limitations that reduce its therapeutic efficacy. Indeed, phenomena such as reduced solubility, lack of gastric stability and hepatic degradation determine a bioavailability of around 30% and a short half-life of ALA. The innovative oral formulation has the potential to overcome these pharmacokinetic limitations as it uses only the R-(+)-lipoic acid, the natural and more active form, whose solubility and stability in gastric environment are ensured by the patented liquid solution. Analysis of the pharmacokinetic profile showed that, with this formulation, the absorption of R-(+)-lipoic acid is accelerated with high plasmatic concentrations and prolonged stability. Therefore, the bioavailability, which tends towards intravenous bioavailability, is markedly greater than that recorded with a solid R-(+)-lipoic acid formulation. To overcome the pharmacokinetic limitations of current solid oral formulations, one potentiates the biological efficacy of ALA: indeed, in animal models, R-(+)-lipoic acid in a liquid formulation amplifies the recovery of the conduction velocity of sensory and motor nerves altered by diabetic neuropathy. These results highlight that the characteristics of R-(+)-lipoic acid in the liquid formulation lead to a more effective and faster biological response suggesting a new therapeutic scenario for numerous oxidative stress-dependent pathologies (diabetic complications, peripheral neuropathies, neurodegenerative and cardiovascular diseases, etc.) requiring chronic treatment.[4]
References
[1] Liu Q, Li W, Huang S, et al. R- Is Superior to S-Form of α-Lipoic Acid in Anti-Inflammatory and Antioxidant Effects in Laying Hens. Antioxidants (Basel). 2022;11(8):1530. Published 2022 Aug 5. doi:10.3390/antiox11081530
[2] Liu J, Song Q, Huang Y, Sun W, Lu D, Zhou B. R-lipoic acid overdosing affects platelet life span via ROS mediated autophagy. Platelets. 2018;29(7):695-701. doi:10.1080/09537104.2017.1356450
[3] Lechner S, Steimbach RR, Wang L, et al. Chemoproteomic target deconvolution reveals Histone Deacetylases as targets of (R)-lipoic acid. Nat Commun. 2023;14(1):3548. Published 2023 Jun 15. doi:10.1038/s41467-023-39151-8
[4] Brufani M, Figliola R. (R)-α-lipoic acid oral liquid formulation: pharmacokinetic parameters and therapeutic efficacy. Acta Biomed. 2014;85(2):108-115. Published 2014 Aug 20.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringN-(2-chloropyrimidin-4-yl)-2,3-dimethyl-2H-indazol-6-amine can be prepared from 2-ethylaniline by nitration, cyclization and methylation reactions.....
Jul 28,2025Pharmaceutical intermediates(R)-LIPOIC ACID
1200-22-2You may like
- Lipoic acid
-

- $50.00 / 1mL
- 2025-12-16
- CAS:1200-22-2
- Min. Order:
- Purity: 99.79%
- Supply Ability: 10g
- (R)-α-Lipoic Acid
-

- $0.00 / 1Kg/Bag
- 2025-12-16
- CAS:1200-22-2
- Min. Order: 1KG
- Purity: 99%HPLC; USP
- Supply Ability: 500KGS
- (R)-(+)-1,2-Dithiolane-3-pentanoic acid
-

- $0.00 / 1kg
- 2025-12-16
- CAS:1200-22-2
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20t