Application researches of Dodecylpyridinium chloride
Sep 29,2025
Introduction
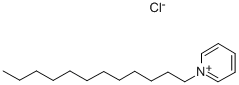
Quaternary ammonium compounds (QACs) are the most common cationic surfactants and can be classified according to the number of quaternary nitrogen atoms in the molecule. These compounds present in their structures an ammonium or pyridinium cation moiety, as observed in dodecylpyridinium chloride (DPC;Figure 1) molecules. Dodecylpyridinium chloride presents a nonpolar hydrocarbon chain ending with a methyl group, nitrogen, and chloride as a counter ion on its molecule. Cationic surfactants such as dodecylpyridinium chloride are used in a wide variety of applications such as in textile softeners, pharmaceuticals, emulsions, disinfectants, and human hair cosmetics.[1]

Dodecylpyridinium chloride as a potentially selective fluorescence quenching agent
Fluorescence behavior is reported for 13 alternant and 12 nonalternant polycyclic aromatic hydrocarbons (PAHs) dissolved in aqueous micellar cetyltrimethylammonium chloride (CTAC)+dodecylpyridinium chloride (DDPC) and sodium dodecylsulfate (SDS)+dodecylpyridinium chloride (DDPC) mixed surfactant solvent media. Experimental measurements indicate that the dodecylpyridinium cation selectively quenches fluorescence emission of alternant PAHs. Emission intensities of nonalternant PAHs, with a few noted exceptions, essentially remain constant, irrespective of both dodecylpyridinium chloride concentration and cosurfactant headgroup charge.
First, it increases the number of mixed surfactant mobile phases that can be used in chromatographic separations that employ fluorescence detection. The miscibility region of mixtures containing both cationic and anionic surfactants is governed to a large extent by the relative sizes of the two alkyl chains. There will be occasions whenever phase miscibility can be achieved simply by substituting dodecylpyridinium chloride for cetylpyridinium chloride and vice versa. Secondly, our observations regarding the fluorescence quenching behavior of DDPy+ suggest that quenching selectivity may extend to even smaller alkylpyridinium cations, perhaps even down to the methylpyridinium cation. This raises the possibility that one might be able to replace the nitromethane selective quenching agent with perhaps a small alkylpyridinium chloride for use with neat acetonitrile or binary aqueous–acetonitrile mixtures.Large inner-filtering corrections are often required in the case of nitromethane, and it would be advantageous to find a selective fluorescence quenching agent that is more optically transparent in the PAHs’ excitation spectral regions.[2]
Complex formation 1
The complex formation between dodecylpyridinium chloride (DPC) and multicharged anionic planar substances, 14 azo dyes and 3 benzene- or naphthalenesulfonates, has been studied by the potentiometric titration using a surfactant selective electrode. The agreement between the observed maximum binding number and the number of anionic charges (n) on dye molecules showed n:1 complex formation. The binding isotherms were found to be composed of two types of binding; one is the noncooperative binding observed at low surfactant concentrations and the other is the cooperative binding at the higher concentrations. The microscopic binding constant for the noncooperative binding was found to take the values in the range of 50-200 dm3/mol for many of the substances, but, takes more large values up to 2500 dm3/mol for the substances which have a large hydrophobic part or the structure of separate hydrophobic and hydrophilic regions. A multiple regression analysis showed that the data of the corresponding standard free energy change of binding were well interpreted by the equation (in unit of kJ/mol) DeltaG degrees=-5.85 logPS-1.68logPD-2.12z+28.4, where PS and PD are the partition coefficients of the surfactants and planar substances in the 1-octanol/water system and z is the number of anionic charges on the planar molecules. At the beginning of the cooperative binding, precipitate formation was observed for almost all of the present systems. Among these, some of the dyes having the structure of separate hydrophobic and hydrophilic regions formed a needle like crystal, which was accompanied by a hysteresis phenomenon in the binding isotherm. The stable complex formation by both the hydrophobic and electrostatic interactions between the surfactant and the planar substances was found to be important for the crystal formation. Depending on the manner of arrangement of the charged groups on the planar substances, the origin of the binding cooperativity was ascribed to the interactions between surfactants bound to one planar-substance molecule or to the association of the complexes. It was also found that the present small binding systems are useful as the model of ligand binding to protein local structures.[3]
Dodecylpyridinium chloride as the collector
Ionic liquids are ionic compounds composed of anions and cations, and they are widely used in chemical reaction solvents,catalysts, as well as electrolyte material fields due to their heat stability, excellent ionic conductivity, and low vapor pressure.Interestingly, ionic liquids were also used as collectors for mineral flotation. Nonetheless,limited work has been carried out on the reverse flotation of muscovite from apatite using ionic liquids as collectors. It is worth noting that the ionic liquid dodecylpyridinium chloride has both cationic and steric characteristics which may have good collecting properties and good foam flow properties for silicate mineral flotation. Now, dodecylpyridinium chloride was used for the first time as the collector in the reverse flotation of muscovite from apatite.
Reverse flotation separation of muscovite from apatite using a dodecylpyridinium chloride (DPDC) ionic liquid as the collector was studied in this work. The microflotation results depicted that dodecylpyridinium chloride had a strong collecting for muscovite but had a slight collecting for apatite when using phosphoric acid as a depressant for apatite in a weakly acidic pH value pulp, artificial mixture mineral flotation showed that reverse flotation separation of muscovite from apatite can be effectively achieved in the reagent scheme of phosphoric acid/dodecylpyridinium chloride, and dodecylpyridinium chloride had a better separation performance in the muscovite/apatite system than DDA. The adsorption measurements indicated that the adsorption amount of dodecylpyridinium chloride on the apatite surface was less than that of dodecylpyridinium chloride on the muscovite surface, and the zeta potential results confirmed that a strong interaction occurred between dodecylpyridinium chloride and the muscovite surface, while an extremely weak interaction occurred between dodecylpyridinium chloride and the apatite surface in the presence of phosphoric acid at pH ∼ 5.5. XPS analysis indicated that a hydrogen bond occurred between dodecylpyridinium chloride and the muscovite surface. Thus, it was inferred that dodecylpyridinium chloride could be used as an appreciation collector for the reverse flotation of muscovite from apatite.[4]
References
1.Nunes RF, Metolina P, Teixeira ACSC. Dodecylpyridinium chloride removal by persulfate activation using UVA radiation or temperature: experimental design and kinetic modeling. Environ Sci Pollut Res Int. 2021;28(48):68229-68243. doi:10.1007/s11356-021-15174-w
2.Pandey S, Roy LE, Acree WE Jr, Fetzer JC. Examination of dodecylpyridinium chloride as a potentially selective fluorescence quenching agent for discriminating between alternant versus nonalternant polycyclic aromatic hydrocarbons. Talanta. 1999;48(5):1103-1110. doi:10.1016/s0039-9140(98)00332-4
3.Murakami K. Complex formation between dodecylpyridinium chloride and multicharged anionic planar substances. Langmuir. 2004;20(19):8183-8191. doi:10.1021/la048965+
4.Liu C, Han S, Zhu Y, Xu W, Yang S. Novel Collector of a Dodecylpyridinium Chloride Ionic Liquid in the Reverse Flotation Separation of Muscovite from Apatite. Langmuir. 2025;41(4):2834-2842. doi:10.1021/acs.langmuir.4c04703
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical Engineering2-Chloro-5-Nitropyridine is an important pyridine derivative, which is applied as fungicides, plant growth regulators and pharmaceutical intermediates.....
Sep 29,2025Pharmaceutical intermediatesDodecylpyridinium chloride
104-74-5You may like
Dodecylpyridinium chloride manufacturers
- Dodecylpyridinium chloride
-

- $0.00 / 1kg
- 2025-11-27
- CAS:104-74-5
- Min. Order: 1kg
- Purity: 99.3%
- Supply Ability: 1000kg
- Dodecylpyridinium chloride
-

- $10.00 / 1KG
- 2025-11-27
- CAS:104-74-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- Dodecylpyridinium chloride
-

- $5.00 / 1KG
- 2025-05-26
- CAS:104-74-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10000kg