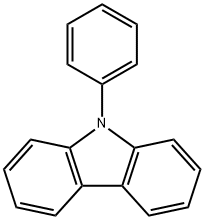
Applications and Properties of N-Phenylcarbazole Hydrochloride
Apr 21,2025
N-Phenylcarbazole hydrochloride is a colourless crystal or light yellow solid with an aromatic odour. It is soluble in organic solvents such as alcohols and ethers at room temperature, but insoluble in water. It is also used as a precursor for organic dyes and electronic materials. N-Phenylcarbazole hydrochloride has been used in the electrochemical synthesis of poly(N-phenylcarbazole) films via direct anodic oxidation in mixed electrolytes of boron trifluoride diethyl etherate and sulfuric acid.

Syhthesis of N-Phenylcarbazole hydrochloride
(1) Coupling reaction process:Under the protection of nitrogen, add carbazole (1.0mol, 167.2g) to a dry 1000mL four-neck round bottom flask equipped with a stirrer, a thermometer, a reflux condenser, andCopper 8-hydroxyquinoline (0.1mol, 35.2g), sodium tert-butoxide (1.0mol, 96.1g),Fluorobenzene (1.1mol, 103.2mL) and N-methylpyrrolidone (500mL), then heated to 50-60°C and stirred for 30 minutes;After the mixture is uniform, the reaction is continued for 10 hours at a heating rate of 3°C/min to 110°C, during which the conversion rate of the carbazole is measured by the liquid phase.After the reaction is complete (the conversion rate of the carbazole detected by the liquid phase is ≥99%), stop the reaction,After the temperature of the reaction system is reduced to 60°C, DMSO is removed by distillation under reduced pressure,Add ethyl acetate to the solid residue to dissolve, filter under reduced pressure, filter cake A is retained, the filtrate is washed with water and extracted,The organic layer extract was distilled under reduced pressure to remove ethyl acetate to obtain a crude product. The crude product was recrystallized from ethanol to obtain 110.9 g of light yellow solid N-Phenylcarbazole hydrochloride (HPLC detection content ≥99%), and the yield was 45.6%.[1]
Probe the effects of ring twist on charge transfer in N-phenylcarbazole hydrochloride
High resolution electronic spectra of 2-phenylindole (PI) and N-phenylcarbazole hydrochloride (PC) have been recorded in the collision-free environment of a molecular beam. Inertial defects determined from fits of the spectra were used to determine the twist angles between the two chromophores and their attached benzene rings in the ground (S0) and excited (S1) electronic states. PI was found to be significantly more planar than N-phenylcarbazole hydrochloride, especially in the S1 state. Stark-effect measurements of the permanent electric dipole moments of both molecules in both states show that significantly more charge is transferred from the phenyl group to the chromophore in PI (0.13e) than in N-phenylcarbazole hydrochloride (0.076e) when the photon is absorbed. Thereby demonstrated for the first time is a direct connection between photo-induced geometry change and charge transfer on excitation of an isolated molecule by light.[2]
In what follows, we establish a connection between the spectroscopic data presented here and the degree of charge transfer in the excited electronic states of PI and PC. First, we note that, while the S1–S0 electronic origin bands of the two molecules are polarized differently, their electronic transition moments (ETMs) are both oriented along the substituent-phenyl bond. The S1–S0spectrum of carbazole is a b-type spectrum, and the S1–S0 spectrum of N-phenylcarbazole hydrochloride is an a-type spectrum, so the ETMs in the two species are oriented similarly in the molecular frame. The ETM of the S1–S0 spectrum of PI is aligned along the phenyl-indole bond, just as PC has its S1–S0 ETM aligned along the phenyl-carbazole bond. So, the direction of charge transfer in the two molecules is the same. But the magnitude of charge transfer is different. The S1–S0 origin band of PC is shifted by ∼1600 cm−1 to the red of the corresponding origin band of PI, reflecting the larger size of the “box” in which the π electrons of N-phenylcarbazole hydrochloride reside. But the substitution of the two parent molecules by phenyl groups also produces red shifts, 742 cm−1 in the case of PC and 3567 cm−1 in the case of PI. The fact that the red shift caused by phenyl substitution is five times larger in PI than in PC, despite the larger size of the PC “box”, shows clearly that the S1–S0 transition in PI results in a substantially larger degree of charge transfer than the corresponding transition in N-phenylcarbazole hydrochloride.
The relation between twisting and charge transfer may be quantified in the following way. The inertial defects of the two states were computed from optimized structures using M05-2X/6-31+(G)* and CIS/6-311(G) methods and fit to 4th order and 5th order polynomials, respectively. Interpolations of these data show that PI has a vibrationally averaged torsional angle of 26.3° in the ground state and a vibrationally averaged torsional angle lying between 12.9 and 18.6° in the excited state, among the vibronic bands analyzed here, and near zero in the zero-point vibrational level of the S1 state. In contrast, the inertial defect of PC is much larger in magnitude than that of PI in the ground state (−118 μÅ2vs. −22 μÅ2) and only slightly smaller in the excited state (−113 μÅ2). The corresponding angles between the ring planes in N-phenylcarbazole hydrochloride were determined (by a similar method) to be 56 and 54°, respectively. Thus, light-induced delocalization of the electrons in N-phenylcarbazole hydrochloride is inhibited by the presence of non-coplanar aromatic rings.
Greenish-blue-emitting iridium dendrimers with N-phenylcarbazole hydrochloride-based polyether dendrons
A series of solution processible greenish-blue-emitting Ir dendrimers with polyether dendrons that consist of N-phenylcarbazole hydrochloride (NPC) are developed via a convenient post-dendronization method. It involves two steps: (i) the successful preparation of a reactive Ir core, namely m-HO-dfppyIr, only when the hydroxyl group is located at the meta position relative to the N atom in the C^N ligand so as to eliminate the possible resonance structure between enol and keto; and (ii) the subsequent functionalization with N-phenylcarbazole hydrochloride-based polyether dendrons to afford the first, second and third generation Ir dendrimers (Ir-G1B, Ir-G2B and Ir-G3B) with ease and high yields over 60%. All these dendritic complexes possess good thermal stability with decomposition temperatures higher than 380 °C and glass transition temperatures higher than 200 °C.[3]
In conclusion, a convenient post-dendronization method is demonstrated for the development of solution processible greenish-blue-emitting Ir dendrimers with polyether dendrons made of N-phenylcarbazole hydrochloride. Since the possible existing resonance structure between enol and keto that is induced by the hydroxyl position has a great effect on the coordination reaction, m-HO-dfppyIr with three reactive hydroxyl groups at the meta position relative to the N atom is successfully synthesized, which is then attached to N-phenylcarbazole hydrochloride -containing polyether dendrons to afford Ir dendrimers of the first, second and third generation (Ir-G1B, Ir-G2B and Ir-G3B) in high yields over 60%.
Preparation and antitumor application of N-phenylcarbazole/triphenylamine
Herein, four N-phenylcarbazole hydrochloride /triphenylamine-appended half-sandwich IrIII salicylaldehyde Schiff base complexes (Ir1–Ir4) were designed and prepared. These complexes exhibited favourable hydrolytic properties, while remaining stable in the condition of bloodstream, which contributed to binding serum albumin with a static quenching mechanism and transport through it. Meanwhile, the weak conjugated structure of the Schiff base ligands (L1 and L2) confirmed that the introduction of N-phenylcarbazole hydrochloride or triphenylamine had little effect on their electron-donating capacity, but the antitumor activity was effectively improved compared to the N-phenylcarbazole hydrochloride /triphenylamine-free complex (Ir5).[4]
Additionally, the introduction of N-phenylcarbazole hydrochloride /triphenylamine endowed the complexes with suitable fluorescence property. Meanwhile, these complexes could effectively inhibit the migration of tumor cells. In summary, N-phenylcarbazole hydrochloride /triphenylamine-appended half-sandwiched IrIII salicylaldehyde Schiff base complexes can induce lysosomal damage and the accumulation of intracellular ROS, lead to apoptosis and inhibit cell migration, showing potential application in the field of organometallic antitumor drugs.
References
[1]SINOSTEEL NEW MATERIALS - CN112079768, 2020, A
[2]Young, J W et al. “Using high resolution electronic spectroscopy to probe the effects of ring twist on charge transfer in 2-phenylindole and N-phenylcarbazole.” Physical chemistry chemical physics : PCCP vol. 15,25 (2013): 10251-7.
[3]Wang, Yang et al. “Synthesis and properties of greenish-blue-emitting iridium dendrimers with N-phenylcarbazole-based polyether dendrons by a post-dendronization route.” Dalton transactions (Cambridge, England : 2003) vol. 44,3 (2015): 1052-9.
[4]Wang, Liyan et al. “Preparation and antitumor application of N-phenylcarbazole/triphenylamine-modified fluorescent half-sandwich iridium(III) Schiff base complexes.” Dalton transactions (Cambridge, England : 2003) vol. 50,43 15888-15899. 9 Nov. 2021
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical Engineering1-Phenoxy-2-propanol (PP) is a glycol ether that is used as a preservative in cosmetics and an effective gastropod anaesthetic.....
Apr 22,2025Chemical ReagentsN-PHENYLCARBAZOLE HYDROCHLORIDE
1150-62-5You may like
N-PHENYLCARBAZOLE HYDROCHLORIDE manufacturers
- N-Phenylcarbazole
-
- $200.00 / 1KG
- 2025-09-25
- CAS:1150-62-5
- Min. Order: 1KG
- Purity: 99%, 99.5% Sublimated
- Supply Ability: g-kg-tons, free sample is available
- N-PHENYLCARBAZOLE HYDROCHLORIDE
-

- $0.00 / 1KG
- 2025-06-27
- CAS:1150-62-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg
- N-PHENYLCARBAZOLE HYDROCHLORIDE
-

- $20.00 / 1kg
- 2025-06-20
- CAS:1150-62-5
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 10 tons