Applications of Methyl propiolate
Nov 15,2019
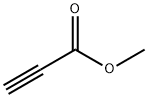
Methyl propiolate(MP) is an organic compound with the formula HC2CO2CH3. It is the methyl ester of propiolic acid, the simplest acetylenic carboxylic acid. It is a colorless liquid that is miscible with organic solvents. The compound is a reagent and building block for the synthesis of other organic compounds, reactions that exploit the electrophilicity of the alkyne group. Methyl propiolate was used in the synthesis of polysubstituted 3-arylaminoacrylate and tetrahydropyrimidin-2-one derivatives. It was also used as a thiol derivatizing agent for capillary electrophoresis.
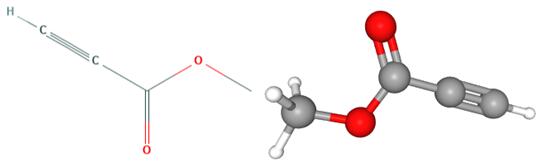
Fig 1. Chemical structure formula and three-dimensional structure of Methyl propiolate
Methyl propiolate is known to react with thioacetamide and thiobenzamide in methanol at 20°C to form E,E- and Z,Ε-bis(methoxycarbonylvinyl) sulfides in a ratio 1:1. Methyl propiolate reacts with thiosemicarbazones in methanol in the presence of triethylamine to give 2-benzylidene(thenylidene, or furfurlidene)azino-3H1,3-thiazin-4-ones[1]. Methyl propiolate is used for N-3 protecting reagent for thymidines and uridines. Methyl propiolate is also used in a one-pot, four-component synthesis of 1,2,3,5-benzenetetracarboxylates promoted by Ph3P[2]. Methyl Propiolate is used as a reagent in the synthesis of a novel class of salicylic acid derivatives for inhibiting the protein tyrosine phosphatase YopH from Yersinia pestis. Several 1-(4-substituted)-phenyl-4- or 5-methoxycarbonyl-1,2,3-
triazoles have been synthesized by 1,3-dipolar cycloaddition of the correspondingarylazides to methyl propiolate in carbon tetrachloride. Polysubstituted 3-arylaminoacrylate and tetrahydropyrimidin-2-one derivatives could be selectively produced from the one-pot domino reaction of arylamines, methyl propiolate, aromatic aldehydes, and urea in ethanol in the presence of FeCl3 as catalyst. Under similar reactions secondary amines such as morpholine and piperidine predominately afford tetrahydropyrimidin-2-one derivatives in good yields[4]. The reaction of Ru(CO)HCl(PPh,), with methyl propiolate gives two bis-insertion derivatives resulting from a head-to-head dimerization reaction. Under high pressure conditions, cycloheptatriene reacts with methyl propiolate to afford mono-, bis- and trisadducts all retaining, the norcaradiene structure.
Methyl Propiolate are lachrymatory liquids; handle in a well-ventilated fume hood.
References
[1] K. A. Volkova, A. S. Nakhmanovich, V. N. Elokhina, et al. Reaction of Dithiomalonic Acid Dianilide with Methyl Propiolate.[J]. 2010, 38(46).
[2] Tetrahedron Lett. 36, 3261, (1995) 2. Helv. Chim. Acta 89, 2918, (2006).
[3] Paudyal, M.P., et al.: Bioorg. Med. Chem., 22, 6781 (2014).
[4] Li-Li Z , Jing S , Chao-Guo Y . Synthesis of tetrahydropyrimidin-2-ones via FeCl3 catalyzed one-pot domino reaction of amines, methyl propiolate, aromatic aldehydes, and urea[J]. Molecular Diversity, 2014, 18(1):79-89.
- Related articles
- Related Qustion
Antioxidant 1010 is a high molecular weight hindered sterically phenolic antioxidant; very low volatility; food contact approval.Antioxidant 1010 is white powder, odorless and no pollution.....
Nov 15,2019Catalyst and AuxiliaryN-Nitrosodimethylamine(NDMA) is a nitrosamine, and it plays a role as a mutagen. N-nitrosodimethylamine is a yellow oily liquid with a faint characteristic odor. Boiling point 151-153°C. N-nitrosodimethylamine can reasonably be expected to....
Nov 15,2019Organic Synthesis IntermediateMethyl propiolate
922-67-8You may like
- Some methylglyoxal related diseases
Oct 16, 2025
- The applications of TPGS in drugs
Sep 22, 2025
- Preparation method and oxidation research of methyl linoleate
Apr 29, 2025
Methyl propiolate manufacturers
- Methyl propiolate
-

- $1.00 / 25KG
- 2025-12-11
- CAS:922-67-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- Methyl propiolate
-

- $0.00 / 25kg
- 2025-12-01
- CAS:922-67-8
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 1000kg
- Methyl propiolate
-

- $0.00 / 1KG
- 2025-06-27
- CAS:922-67-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg