How to prepare Bis(pinacolato)diboron?
Dec 24,2019
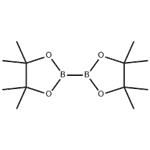
Bis(pinacolato)diboron is a covalent compound containing two boron atoms and two pinacolato ligands. It has the formula [(CH3)4C2O2B]2; the pinacol groups are sometimes abbreviated as "pin", so the structure is sometimes represented as B2pin2. It is a colourless solid that is soluble in organic solvents. It is a commercially available reagent for making pinacol boronic esters for organic synthesis. Unlike some other diboron compounds, B2pin2 is not moisture-sensitive and can be handled in air [1].
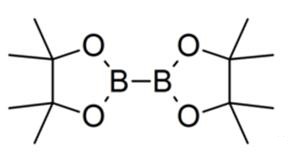
This compound may be prepared by reacting tetrakis(dimethylamino)diboron with pinacol in acidic conditions.[1] The B-B bond length is 1.711(6) Å [2].
The B-B bond adds across alkenes and alkynes to give the 1,2-diborylated alkanes and alkenes. Using various organorhodium or organoiridium catalysts, it can also be installed onto saturated hydrocarbons [3]:
CH3(CH2)6CH3 + [pinB]2 → pinBH + CH3(CH2)7Bpin These reactions proceed via boryl complexes [4].
The addition of diborons to alkenes and alkynes is an attractive and straightforward method to introduce boryl groups into organic molecules. Although tetra(alkoxo)diborons such as bis(pinacolato)diboron are highly inert to ionic reactions, they oxidatively add to a low-valent transition metal with the B–B bond cleavage, thus allowing the catalyzed addition of the diboron to unsaturated organic substrates. The platinum (0) complexes were recognized to be the most efficient catalyst for such diboration of alkenes, alkynes, and conjugate dienes. Kou Takahashi etc. reported the addition reaction of bis(pinacolato) diboron to α, β-unsaturated ketones, esters, or nitriles, and terminal alkynes, and the coupling reaction with allyl chloride mediated by CuX and AcOK [5].
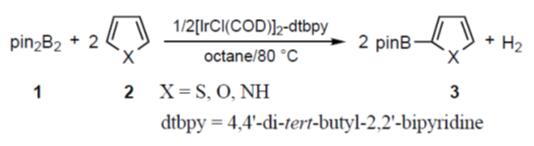
The transition metal-catalyzed cross-coupling reaction of aromatic substrates with (alkoxo)diborons has attracted considerable attention as a powerful tool for the synthesis of arylboron compounds. Aryl electrophiles such as halide and triflate derivatives have been efficiently employed as substrates for this protocol. Although the utility of the reaction has been amply demonstrated by elegant synthesis of natural products, biologically active compounds, and functional materials, an extension of the methodology toward the direct borylation of aromatic hydrocarbons would provide a more efficient and convenient route to arylboron compounds because of the wide availability and low cost of hydrocarbons. Hartwig has reported the C–H coupling of benzene with bis(pinacolato) diboron (pin2B2, pin=Me4C2O2) catalyzed by Cp*Re(CO)3 or Cp*Rh(η4-C6Me6) under photoirradiation or at temperatures above 150°C. Similar reactions with pinacolborane in the presence of a (η5-C9H7)Ir(COD)-dppe (-dmpe) or a (Cp*RhCl2)2 catalyst also have been presented by Smith and Marder, respectively. On the other hand, Jun Takagi etc. found that iridium(I) complexes generated from an air-stable, commercially available [IrCl(COD)]2 precursor and a simple 2,2’-bipyridine (bpy) ligand exhibited high catalytic activity toward the C–H coupling of various arenes with pin2B2 at 80°C. We disclosed the C–H coupling between pin2B2 and heteroaromatic substrates catalyzed by 1/2[IrCl(COD)]2-(4,4’-di-tertbutyl-2,2’-bipyridine) (dtbpy) in octane at 80–100°C to give the corresponding heteroarylboronates in high yields with high regioselectivity [6].
References
[1] Tatsuo Ishiyama; Miki Murata; Taka-aki Ahiko; Norio Miyaura (2004). "Bis(pinacolato)diboron". Organic Syntheses.; Collective Volume, 10, p. 115
[2] C.Kleeberg, A.G.Crawford, A.S.Batsanov, P.Hodgkinson, D.C.Apperley, Man Sing Cheung, Zhenyang Lin, T.B.Marder "Spectroscopic and Structural Characterization of the CyNHC Adduct of B2pin2 in Solution and in the Solid State" J. Org. Chem. 2012, vol. 77, pp. 785. doi:10.1021/jo202127c
[3] Xinyu Liu "Bis(pinacolato)diboron" Synlett 2003, pp 2442–2443. doi:10.1055/s-2003-43344
[4] https://en.wikipedia.org/wiki/Bis(pinacolato)diboron
[5] Kou Takahashi, Tatsuo Ishiyama, and Norio Miyaura, Addition and Coupling Reactions of Bis(pinacolato)diboron Mediated by CuCl in the Presence of Potassium Acetate, Chemistry Letters 2000, 982-983.
[6] Jun Takagi, Kazuaki Sato, John F. Hartwig, Tatsuo Ishiyama, and Norio Miyaura, Iridium-catalyzed C–H coupling reaction of heteroaromatic compounds with bis(pinacolato)diboron: regioselective synthesis of heteroarylboronates, Tetrahedron Letters 43 (2002) 5649–5651.
- Related articles
- Related Qustion
- Bis(pinacolato)diboron: The Invisible Assistant of the Chemical World Jun 14, 2024
This review aims to introduce the properties, synthetic methods, uses, and market development of Bis(pinacolato)diboron
- What is Bis(pinacolato)diboron? Feb 24, 2020
Bis(pinacolato)diboron appears as powder or platelets. Bis(pinacolato)diboron is soluble in tetrahydrofuran, dichloromethane, toluene, hexane and heptane and insoluble in water.
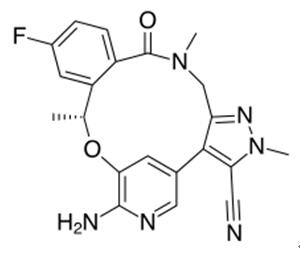
Lorlatinib is a small molecule inhibitor of ALK and ROS1 kinase developed by Pfizer for the treatment of ALK-positive non-small cell lung cancer. It has a role as an antineoplastic agent and an EC 2.7.10.1 inhibitor.....
Dec 24,2019InhibitorsCaffeic acid (CA, 3,4-dihydroxy-cinnamic acid) is an organic compound that is classified as a hydroxycinnamic acid. This yellow solid consists of both phenolic and acrylic functional groups, and it is found in most fruit, e.g., kiwis [1]. C....
Dec 24,2019APIBis(pinacolato)diboron
73183-34-3You may like
Bis(pinacolato)diboron manufacturers
- Bis(pinacolato)diboron
-
- 2025-12-13
- CAS:73183-34-3
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Bis(pinacolato)diboron
-

- $0.00 / 25kg
- 2025-12-12
- CAS:73183-34-3
- Min. Order: 1g
- Purity: 99%
- Supply Ability: 50mt
- Bis(pinacolato)diboron
-

- $9.90 / 1KG
- 2025-12-11
- CAS:73183-34-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 5tons