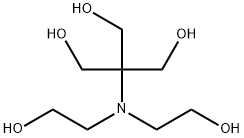
Bis-Tris Methane: Zwitterionic Buffer & Protein Separation Optimization
Dec 17,2025
Bis-tris methane, also known as BTM, is a zwitterionic buffering agent widely employed in biochemical and biological research. It functions effectively in aqueous solutions within a pH range of 5.8 to 7.2 and demonstrates minimal shifts in its dissociation constant. Further, it can be used as a substitute for the highly toxic buffer cacodylate and is also recognized for its ability to form complexes with different metals. Bis-Tris gels offer a solution to smeary bands and low-resolution issues in SDS-PAGE by providing sharper bands. They are ideal for low molecular weight proteins, Western blots, and tricky samples. Prepare them the same way as SDS-PAGE gels, swapping in it for Tris, and use MES or MOPS in your running buffer depending on the size of your target proteins. Though more expensive, Bis-Tris gels deliver narrower bands with less background staining, making them perfect for crucial experiments.

A Contemporary Twist on the Classic Western Blot Analysis
The Laemmli system for separating a broad range of proteins using SDS-PAGE is the most widely used gel system for Western blotting. Despite the popularity of this Western blotting system, this method can result in band distortion, loss of resolution, and spurious bands. This may be a consequence of the deamination and alkylation of proteins due to the high pH (9.5) of the separating gel, reoxidation of reduced disulfide bonds due to the varying redox state of the gel, and cleavage of aspartyl-prolyl peptide bonds due to heating the protein in Laemmli buffer (pH 5.2). The Bis-Tris gel system, which operates at a neutral pH (7.0), provides significant benefits over the Lamemmli system. This system improves protein stability, minimizes protein modifications, maintains proteins in their reduced states, prevents aspartyl-prolyl cleavage during electrophoresis, and more importantly, the electrophoresis run time is 35 min. In addition, the Bis-Tris gel system also produces sharper bands, higher resolution and separation, and increased sensitivity resulting in more reliable data. In conjunction with the Bis-Tris gel system, the iBlot dry blotting system uses high field strength and currents to significantly reduce the transfer time of proteins from gels onto membranes within 7 min. This transfer system is based on the dry blotting method that generates a more efficient and reliable transfer of proteins.[1]
Lysates of two-fold serial dilutions of WM793 human-derived melanoma protein were separated using a NuPAGE Novex Bis-Tris gel with protein transferred onto PVDF membrane using the iBlot transfer apparatus. Membranes were blocked in Odyssey Blocking Buffer and probed with the following primary antibodies: rabbit anti-total ERK1/2, rabbit anti-total BIM, and mouse anti-β-Actin. if a Tris-Glycine SDS Sample Buffer is used instead of the LDS Sample Buffer, this will lead to the cleavage of the aspartyl-prolyl bonds, a decrease in the sharpness of the protein bands, and an increase protein fragmentation. It is also pertinent to add the Antioxidant to the Upper Buffer Chamber of the Mini-Cell electrophoresis unit. Since the Antioxidant is able to comigrate with the samples at neutral pH in the Bis-Tris gel system, this can help maintain proteins in a reduced state and protect disulfide bonds as well as sensitive amino acids against oxidation, whereas the absence of the Antioxidant can lead to diffused protein bands. Lastly, it is highly recommended to use either MES or MOPS Running Buffer with Bis-Tris gels as opposed to using Tris-Glycine SDS Running Buffer with these types of gels. The combination of Tris-Glycine SDS Running Buffer and Bis-Tris gels results in the slow migration of glycine ions causing a dramatic increase in the electrophoresis run time with bands appearing more compressed and cup-shaped.
Models of protein modification in neutral pH Bis–Tris gels
Alternative gels and running conditions where the pH is more favorable have been described and are commercially available. The commercial gels are formulated with Bis-Tris chloride as the gel buffer at neutral pH. The running buffer is 50 mM Mes with 50 mM Tris at pH 7.2 or Mops and Tris at pH 7.7. The ion trailing the chloride toward the anode as voltage is applied is Mes or Mops instead of glycine as in the Laemmli gels. Protein is exposed to Bis–Tris, Mes or Mops, and Tris ions as the Tris in the lower tank buffer migrates to displace the cathodally migrating Bis-tris. Under these near neutral pH conditions, the reactivity of the amino and sulfhydryl groups should be diminished. Sample buffer is also set higher at pH 8.5 to avoid acidification of the buffer during heating. Using model systems and kinetic methods, we compare the rates of reaction of sulfhydryl and amino groups with those of acrylamide under conditions that simulate the environment to which proteins would be exposed during separation on polyacrylamide gels using the conventional Laemmli buffer system or the Bis–Tris–Mes buffer system. We determine rate constants that allow the calculation of actual half-lives for reactive functional groups so that the extent of reaction can be predicted for other conditions. Geisthardt and Kruppa examined the reactivity of several buffer components with acrylamide, but they did not include all of the components of the Bis-tris gel system and did not obtain rate constants.[2]
During electrophoresis, the buffer composition within the gel changes as the ions move in the applied field. As a result, the pH inside the gel is neither the same as the pH of the buffer cast originally in the gel nor the same as the pH of the running buffers in the reservoirs. To determine the pH to which proteins inside the gel are exposed, we ran gels using the Bis-tris-Mes and Tris–glycine systems, cut the gels into strips perpendicular to the electric field, soaked them in water, and measured the pH. In the Bis-tris-Mes system, the pH of the running buffer is 7.2, but we found that it varies from 6.75 at the top of the gel to 7.5 at the anodic end. The pH of the Tris–glycine running buffer is 8.3. We have demonstrated that the environment to which proteins are exposed in conventional Tris–glycine gels is a higher pH than is often recognized. At this pH range, the possibility of protein modification, especially by alkylation of cysteine sulfhydryls, is very significant, even though the concentration of free acrylamide found in commercially produced gels is significantly lower than has been reported for handcast gels.
References
[1]Silva JM, McMahon M. The fastest Western in town: a contemporary twist on the classic Western blot analysis. J Vis Exp. 2014 Feb 5;(84):e51149. doi: 10.3791/51149. PMID: 24561642; PMCID: PMC4028330.
[2]Hachmann, John P, and Joseph W Amshey. “Models of protein modification in Tris-glycine and neutral pH Bis-Tris gels during electrophoresis: effect of gel pH.” Analytical biochemistry vol. 342,2 (2005): 237-45. doi:10.1016/j.ab.2005.04.015
- Related articles
- Related Qustion
L-(+)-Ergothioneine (EGT) is a potent, non-toxic, and highly stable antioxidant synthesized by fungi, algae, and bacteria, but not by animals or higher plants.....
Jan 28,2026SupplementsN,N-Diethylnicotinamide acts as a ligand for rare earth/copper complexes, and a hydrotropic agent accelerating PTX release from PLGA matrices.....
Dec 17,2025Chemical MaterialsBIS-Tris
6976-37-0You may like
- BIS-TRIS
-

- $0.00 / 1kg
- 2026-02-03
- CAS:6976-37-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20MT
- BIS-TRIS
-

- $0.00 / 1kg
- 2026-02-03
- CAS:6976-37-0
- Min. Order: 1kg
- Purity: 99%-101%
- Supply Ability: 500 KG
- BIS-Tris
-
- $5.00 / 1KG
- 2025-09-25
- CAS:6976-37-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available