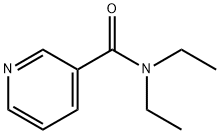
N,N-Diethylnicotinamide: Ligand for Metal Complexes and Paclitaxel Hydrotropic Agent
Dec 17,2025
N,N-Diethylnicotinamide (DENC) is commonly used as a ligand to prepare tetranuclear copper complexes. As a classic respiratory centre stimulant, it is primarily used in emergency treatment for central respiratory and circulatory failure, and may also counteract poisoning from central nervous system depressants such as anaesthetics. Animal studies indicate that doses of 10–500 mg/kg improve respiratory function and survival rates in mice following microwave irradiation. Furthermore, N,N-Diethylnicotinamide serves as a precursor for synthesising tetranuclear copper complexes, finding extensive application in scientific research. This substance is typically synthesised by dehydrating the salt formed from nicotinic acid and diethylamine, achieving a purity exceeding 99%. It must be stored sealed in a cool, dry environment.

Aqueous N,N-diethylnicotinamide solution as a medium for accelerated release
N,N-Diethylnicotinamide was identified as an excellent hydrotropic agent for paclitaxel (PTX) in our previous studies. The aqueous solubility of PTX was increased by several orders of magnitude in the presence of DENA. Because of such a high hydrotropic property, DENA was used as a release medium providing a sink condition for the release of PTX from poly(lactic-co-glycolic acid) (PLGA) matrices. The release profiles of PTX from PLGA matrices into DENA, serum and phosphate-buffered saline (PBS) were compared. The stability of PTX in DENA and the degradation of PLGA molecules in DENA were examined. The degradation rate constant of PTX in 2 M N,N-Diethylnicotinamide was similar to those in other aqueous solutions. The use of 2 M DENA as a release medium allowed differentiation of the release profiles of PTX from PLGA matrices made of different PLGA compositions.[1]
The PTX release from PLGA matrices was much faster in DENA solution than in serum or PBS, and the concentration of N,N-Diethylnicotinamide affected the PTX release rate. The presence of DENA in the release medium increased the hydrolysis rate of PLGA polymers. The faster release of PTX from PLGA matrices in DENA solution may be due to the high PTX solubility and faster degradation of PLGA polymers in the presence of DENA. Our study suggests that the aqueous N,N-Diethylnicotinamide solution can be used for the accelerated release study of PTX from PLGA matrices.
Novel mixed ligand coordination compounds containing acesulfamato/N,N-diethylnicotinamide
Studies on the coordination compounds of rare earth metal (REM) cations started later than studies on transition metals. The complex compounds formed by rare earth metal cations with organic ligands have rich chemistry, unique physical properties, different and essential application areas. For this reason, interest in these compounds has been increasing for the last half-century. In this study, coordination compounds with mixed ligands of acesulfame ( acs ) and N,N-Diethylnicotinamide (nikethamide, dena ) of the rare earth metal cations Eu3+, Tb3+, Ho3+, Er3+, and Yb3+ were synthesized. Afterwards, the structural characterizations of the molecules were examined, and their thermal degradation steps were investigated in detail. The potassium perchlorate (KClO4) was expected to settle from the continuously stirred solution. Some cold ethyl alcohol was added to the solution to make the precipitation process better. After precipitation, filtration was carried out, and 50 mL of an aqueous solution of 0.02 mol of nicotinamide was added to the solution. The total solution mixed over a magnetic stirrer for 2 h was stored at room conditions for crystallization. The visualization of two-stage general synthesis reactions of mixed ligand metal-acesulfame/ N,N-Diethylnicotinamide complexes is given.[2]
Complex-ligand complexes of rare earth metal cations containing acesulfamato/ N,N-diethylnicotinamide were synthesized. Analysis techniques such as elemental analysis, Fourier Transform infrared spectroscopy (FTIR), solid ultraviolet-visible spectroscopy (UV-vis), mass spectroscopy (GC-MS) were studied for the structural properties of the complexes. With the recorded thermal analysis (TGA / DTA) curves, comments were made on the degradation steps of molecules and possible degradation products. In the structure of complexes, depending on the steric obstacle, two acesulfamato ligands showed monoanionic-monodentate coordination through acidic -N group, while two N,N-diethylnicotinamide ligands provided coordination with the metal through the strong electron donor-N- group in the pyridine ring. The M(III) cations, which are predicted to exhibit sixth coordination, complete the coordination circles with two moles of aqua ligands entering the coordination sphere.
References
[1]Baek, Namjin et al. “Aqueous N,N-diethylnicotinamide (DENA) solution as a medium for accelerated release study of paclitaxel.” Journal of biomaterials science. Polymer edition vol. 15,4 (2004): 527-42. doi:10.1163/156856204323005343
[2]Zeybel L, Köse DA. Novel mixed ligand coordination compounds of some rare earth metal cations containing acesulfamato/N,N-diethylnicotinamide. Turk J Chem. 2021 Aug 27;45(4):1004-1015. doi: 10.3906/kim-2012-47. PMID: 34707430; PMCID: PMC8520393.
- Related articles
- Related Qustion
Bis-Tris (zwitterionic buffer) works at pH 5.8-7.2, optimizes SDS-PAGE/Western blot with sharp bands, and substitutes toxic cacodylate.....
Dec 17,2025Chemical MaterialsN,N-DIETHYLNICOTINAMIDE
59-26-7You may like
N,N-DIETHYLNICOTINAMIDE manufacturers
- N,N-DIETHYLNICOTINAMIDE
-

- $0.00 / 1kg
- 2025-12-16
- CAS:59-26-7
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 1000kg
- N,N-DIETHYLNICOTINAMIDE
-

- $10.00 / 1KG
- 2025-12-11
- CAS:59-26-7
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- N,N-Diethylnicotinamide/?Nikethamide
-

- $0.00 / 1kg
- 2025-07-08
- CAS:59-26-7
- Min. Order: 0.10000000149011612kg
- Purity: 99.0%min
- Supply Ability: 200kg