Dodecyl Acrylate: Synthesis, Applications in Lubricants, and Diffusion-Tension Studies
Apr 28,2025
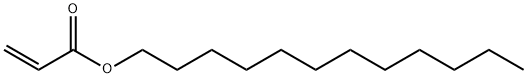
Dodecyl acrylate is a chemical compound with the formula C15H28O2 and the CAS registry number 2156-97-0. It is also known as n-lauryl acrylate, acrylic acid dodecyl ester, n-Dodecyl acrylate and Acrylic acid.

Polyacrylate-magnetite nanocomposite as a potential multifunctional additive for lube oil
The application of polymer nanocomposites (PNCs) in lubricant industry has attracted considerable interest due to their much enhanced properties compared to neat polymers. In this study, magnetite (Fe3O4) nanoparticles (NPs) were synthesized. Then PNCs were prepared by reinforcing these NPs in the homopolymer of dodecyl acrylate in different percentages. Dodecyl acrylate (DDA) was prepared by esterification of acrylic acid with dodecyl alcohol in 1.1:1 mol ratio. The reaction was performed in a resin kettle in presence of concentrated sulfuric acid as a catalyst, 0.25% hydroquinone with respect to the reactants as polymerization inhibitor, and toluene as a solvent. The reaction was carried out under nitrogen atmosphere. The reaction mixture was heated gradually from room temperature to 403 K using a well-controlled thermostat. The extent of reaction was followed by monitoring the amount of water liberated during reaction. After completion of the reaction, the ester dodecyl acrylate (DDA) was collected.[1]
The polymerization was carried out in a four-necked round bottom flask fitted with a condenser, stirrer, thermometer and an inlet for the nitrogen insertion. Required amounts of dodecyl acrylate and initiator (AIBN, 0.5% w/w) were taken in the flask and toluene was also added as solvent. The reaction temperature was controlled at 353 K for 6 h. Then the reaction mixture was poured into methanol solvent with stirring to cease the polymerization and a precipitate was appeared. The precipitated polydodecyl acrylate (A), PDDA, was further purified by frequent precipitation of its hexane solution with methanol followed by drying under vacuum at 313 K.
The FT-IR spectrum of homopolymer of dodecyl acrylate (A) and one of the PNCs (polymer composite F-3) is shown. The absorption band at 1734.56 cm-1 indicated the ester carbonyl stretching vibration of polymer A. The polymer/Fe3O4 nanocomposite showed the absorption for ester carbonyl group at 1726.12 cm-1. This shifting of carbonyl stretching frequency may be due to some association of nanomagnetite and poly dodecyl acrylate. In this study nano-Fe3O4 was synthesized and used as filler particles in the formation of polydodecyl acrylate composites. The characterizations of both the polymer and composites were studied by SEM, TEM, XRD and spectral analysis techniques (FTIR, NMR). Through TGA data we showed that thermal stability of polymer was improved significantly by the incorporation of nanomagnetite into the polymer matrix. During their evaluation as additive for lubricant, it was found that all the nano-blended composites showed improved performance as viscosity modifier and anti-wear additives for lube oil. Therefore, the above study is definitely a potential approach to design multifunctional additives for lubricating oil.
Measuring the Mutual Diffusion Coefficient for Dodecyl Acrylate
Diffusion of small molecules into glassy polymers is quite complicated and almost always non-Fickian. Little work has been done with the diffusion of low molecular weight polymers that are liquids at room temperature (such as poly(dodecyl acrylate)) into their miscible monomers. We have studied three molecular weights under 20 000 to determine if poly(dodecyl acrylate) diffusion into dodecyl acrylate could be treated with Fick's law and if so to determine the values of the diffusion coefficients. We compare two methods for measuring the diffusion of dodecyl acrylate into poly(dodecyl acrylate): We used laser line deflection (Wiener's method) and improved upon the method from published reports. We also used the dependence of pyrene's fluorescence on the viscosity to measure the concentration distribution, and thus to extract the diffusion coefficient. After an initial relaxation period, diffusion in all cases followed Fick's law with a single concentration-independent diffusion coefficient. Comparison of the diffusion coefficients obtained by both methods yielded the same order of magnitude for the diffusion coefficients (10-7 cm2/s) and showed the same trend in the dependence on the average molecular weight of the polymer (a decrease in the diffusion coefficient with an increase in the molecular weight).[2]
To run the diffusion experiments with dodecyl acrylate (DDA) and poly(dodecyl acrylate) (PDDA),we prepared three PDDA samples of different molecular weights. All samples were prepared with dodecyl acrylate (Sartomer, 90%, n = 1.440) as the monomer, Irgacure 184 (Ciba) as a photoinitiator, and dodecyl mercaptan (Sigma Aldrich, 98%) as a chain transfer agent to control the molecular weight. The three samples were made with various concentrations of dodecyl mercaptan in DDA (0.7%, 1.3%, and 2%), 3 × 10-3 M Irgacure, and 10-5 M pyrene (Fluka, 99.0%). The samples were irradiated at 365 nm for 90 min with an EFOS Novacure 100-W mercury vapor light source with an intensity of 45 mW cm-2. The molecular weights of the samples were obtained via gel-permeation chromatography (GPC) calibrated with polystyrene standards and with THF as the mobile phase. Representative samples from each of the three molecular weights of the polymers were examined with an Atago Multi-Wavelength Abbe refractometer DR-M2 to determine a refractive index of 1.464, which indicates independence of the refractive index from molecular weight.
The Existence of an Effective Interfacial Tension between Miscible Fluids Dodecyl Acrylate−Poly(dodecyl acrylate)
In this paper we report our efforts to determine whether a measurable EIT exists between a monomer, dodecyl acrylate (DDA), and its polymer with which it is miscible in all proportions. We used spinning drop tensiometry. We estimated the value of the square gradient parameter for this system and determined its dependence on temperature, concentration, and molecular weight. We prepared four samples of poly(dodecyl acrylate) (pDDA) of different molecular weights. All samples were prepared using dodecyl acrylate (Sartomer, 90%), Irgacure 184 (Ciba) as a photoinitiator, and dodecyl mercaptan (Sigma Aldrich, 98%) as a chain transfer agent to control the molecular weight. The samples were irradiated at 365 nm using an EFOS Novacure 100 W mercury vapor light source.[3]
We studied drops of dodecyl acrylate in poly(dodecyl acrylate) (molecular weight of 25 000) in a spinning drop tensiometer to determine whether an effective interfacial tension (EIT) existed between these two miscible fluids. We found convincing evidence. We estimated the mechanical relaxation time from an immiscible analogue (1-propanol and poly(dodecyl acrylate)) and showed that the dodecyl acrylate drops maintained quasi-steady diameters long after this relaxation period. Drops continuously grew in length and became more diffuse, but the width of the transition zone did not grow with t1/2 as expected from Fick's law although this system had been shown to follow Fick's law in a static configuration. The EIT was determined from Vonnegut's equation, EIT = (Δρ)ω2r3/4; both the inner and outer diameters were measured, yielding values of 0.002 and 0.02 mN m-1, respectively. The EIT was found to be independent of the rotation rate above 6000 rpm and independent of the initial drop volume. The EIT was found to decrease with temperature and increase with the difference in concentration between the monomer drop and polymer−monomer fluid. The square gradient parameter, k, was determined from EIT = k(Δc2/δ), where Δc is the difference in mole fraction and δ is the width of the transition zone. The square gradient parameter was on the order of 10-9 N. The square gradient parameter was found to decrease with temperature, to be independent of concentration, and to increase with the molecular weight of the polymer.
References
[1]Dey K, Karmakar G, Upadhyay M, Ghosh P. Polyacrylate-magnetite nanocomposite as a potential multifunctional additive for lube oil. Sci Rep. 2020 Nov 5;10(1):19151.
[2]Antrim D, Bunton P, Lewis LL, Zoltowski BD, Pojman JA. Measuring the mutual diffusion coefficient for dodecyl acrylate in low molecular weight poly(dodecyl acrylate) with laser line deflection (Wiener's Method) and the fluorescence of pyrene. J Phys Chem B. 2005 Jun 16;109(23):11842-9.
[3]Zoltowski B, Chekanov Y, Masere J, Pojman JA, Volpert V. Evidence for the existence of an effective interfacial tension between miscible fluids. 2. Dodecyl acrylate-poly(dodecyl acrylate) in a spinning drop tensiometer. Langmuir. 2007 May 8;23(10):5522-31.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringPlecanatide is used to treat chronic idiopathic constipation which works by increasing fluid in the intestines and helping food move through the gut.....
Apr 29,2025APIDodecyl acrylate
2156-97-0You may like
Dodecyl acrylate manufacturers
- Dodecyl acrylate
-

- $10.00 / 1KG
- 2025-12-11
- CAS:2156-97-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100 mt
- Lauryl acrylate
-

- $0.00 / 200kg
- 2025-11-19
- CAS:2156-97-0
- Min. Order: 20kg
- Purity: 95.0%
- Supply Ability: 10 tons
- Dodecyl acrylate
-
- $100.00 / 1KG
- 2025-09-25
- CAS:2156-97-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available