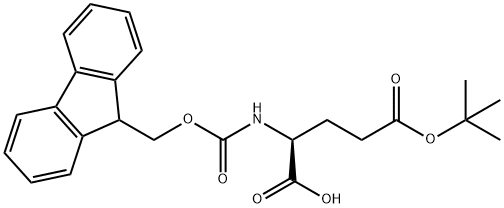
Fmoc-Glu(OtBu)-OH: Self-Assembly, Drug Delivery, and Supramolecular Hydrogel Applications
Sep 28,2025
Introduction
The Fmoc-Glu(OtBu)-OH is a Fmoc derivative with an N-substituted tert‑butyl ester molecule. Fmoc group molecules were reported to have a stable structure, were widely used reagents for a variety of chemical formations, and are important intermediates in the synthesis of pharmaceuticals due to their ability to protect amine groups. The title chemical Fmoc-Glu(OtBu)-OH, also reacted effectively with a wide range of organic compounds in drug delivery systems for various diseases, including Alzheimer's, tuberculosis (TB), cancer, viral and bacterial, inflammatory and was most commonly seen in anti-cancer drug delivery. Furthermore, the title molecule and its derivatives have the potential to self-assemble into nano and microscale structures spontaneously. These Fmoc materials are more significant in bio-chemicals and pharmaceuticals because of their low molecular weight, good stability, high aromatic content, low toxicity, minimal side effect, and amine group protecting properties. Also, the self-assembled Fmoc variants could be used in a range of applications such as supramolecular electronics, drug delivery vectors, photonics, and healing agents in regenerative drugs, cell culture, tissue engineering, and other areas of bionanotechnology. [1]

Self-assembled Structure
Herein, studies report the self-assembled structure formed by Fmoc protected charge singleamino acid Fmoc-L-glutamic acid 5-tert-butyl ester (Fmoc-Glu(OtBu)-OH). The self-assembled architecture formed by the charge aliphatic aminoacids were assessed under different conditions such as concentration, temperature and pH. Fmoc-Glu(OtBu)-OH assembled to spheres at both lower and higher concentration under room temperature condition. However, it forms a broom stick like morphology at both lower and higher concentration on heating. Fmoc-Asp(OtBu)-OH on the other hand formed rod like at both low and high concentration and also on healing. Fmoc-Lys(Boc)-OH also self-assemble to sphere like morphology in all conditions irrespective of concentration and heating. The self-assembled structures formed by modified amino acids are easy and facile route to design novel nanoarchitectures which may be potentially useful in future for various type of applications in the field of material chemistry, bioscience, biomedical. [2]
Fmoc-Glu(OtBu)-OH for Targeted Cancer Therapy Prodrugs Synthesis
Paclitaxel (PTX) is one of the most effective chemotherapeutic drugs ever developed and is effective against a wide spectrum of tumors. The clinical application of PTX, however, is limited by its severe side effects. Studies developed new ligand-mediated prodrugs, namely, PTX conjugated with Fmoc-Glu(OtBu)-OH (linker) and transferrin (Tf, ligand/carriers), to specifically target tumor cells/tissues. Tf–Glu–PTX increased tumor-targeting capability and decreased toxicity to normal cells. It is also potential to circumvent paclitaxel resistance in cancer cells. In vitro and in vivo studies demonstrated that PTX-conjugated Tf carriers shown higher apoptosis and stronger inhibition rate in drug-resistant MDA-MB-231 tumor cells than the free drug. The toxicity study and treatment experiment indicated that PTX-conjugated Tf drug delivery system could significantly decrease organ toxicity compared to free PTX. Taken together, the PTX-conjugated Tf carrier is a prospective targeting drug delivery system for tumor therapy. [3]
Transformations in Supramolecular Hydrogels
To mimic the activity of natural enzymes, control over the reactivity of reactants (or intermediates) is necessary for artificial catalytic systems in aqueous media. Supramolecular hydrogels could be such organized media, where the fibrillar hydrogel network can provide active surface area with efficient diffusion properties and alter the selectivity of aqueous chemical reactions. Fores et al. developed a strategy to grow a catalytically active supramolecular hydrogel in an open-cell melamine foam. An enzyme, alkaline phosphatase, was installed in the porous wall of the foam, which can catalyze in situ formation of the Fmoc-GFFYGHY peptide hydrogelator (OC-9) from the bis-phosphorylated peptide, resulting the formation of the hydrogel network inside the polymer foam. Besides, Fmoc-Glu(OtBu)-OH were converted to the corresponding carboxylic acids using this hydrogel network. The hydrogel could also be used for kinetic resolution by isolating a quantitative amount of enantiopure l-carboxylic acids from Fmoc-Glu(OtBu)-OH. [4]
References:
[1] Thirunavukkarasu, M., Balaji, G., Prabakaran, P., Basha, S. J., Irfan, A., Javed, S. S., & Muthu, S. (2022). Spectral characterization, solvation effects on topological aspects, and biological attributes of Fmoc-L-glutamic acid 5‑tert‑butyl ester: An effective reagent in anticancer evaluations. Journal of Molecular Structure, 1269, 133793.
[2] Gour, N., Koshti, B., Naskar, S., Kshtriya, V., & Narode, H. (2021). Controlled aggregation properties of modified single amino acids.
[3] Shan, L., Shan, X., Zhang, T., Zhai, K., Gao, G., Chen, X., & Gu, Y. (2016). Transferrin-conjugated paclitaxel prodrugs for targeted cancer therapy. RSC Advances, 6(81), 77987-77998.
[4] Das, N., & Maity, C. (2023). Chemical transformations in supramolecular hydrogels. ACS Catalysis, 13(8), 5544-5570.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringGallic acid monohydrate exhibits antibacterial and antiviral activities, its dehydration dynamic, crystal structure and THz-TDS investigation were reported.....
Sep 28,2025SupplementsFmoc-L-glutamic acid 5-tert-butyl ester
71989-18-9You may like
Fmoc-L-glutamic acid 5-tert-butyl ester manufacturers
- Fmoc-L-Glu(OtBu)-OH
-
- $0.00 / 1kg
- 2025-12-12
- CAS:71989-18-9
- Min. Order: 1kg
- Purity: 98+
- Supply Ability: 1T
- Fmoc-Glu(OtBu)-OH
-

- $0.00 / 25kg
- 2025-12-12
- CAS:71989-18-9
- Min. Order: 1kg
- Purity: 98%min
- Supply Ability: 1000kg
- Fmoc-L-glutamic acid 5-tert-butyl ester
-

- $5.00 / 25kg
- 2025-12-12
- CAS:71989-18-9
- Min. Order: 1kg
- Purity: ≥99%
- Supply Ability: 100mt/year