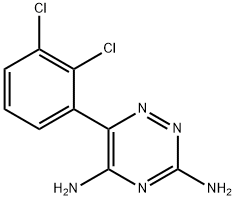
Lamotrigine: Applications in Bipolar Disorder Maintenance and Epilepsy Treatment
May 8,2025
Lamotrigine is an anti-epileptic medication, also called an anticonvulsant. Lamotrigine is used alone or with other medications to treat epileptic seizures in adults and children. Lamotrigine is also used to delay mood episodes in adults with bipolar disorder (manic depression).

Lamotrigine in the maintenance treatment of bipolar disorder
Bipolar disorder is a chronic mental disorder with repetitive mania/hypomania as well as depressive episodes, which eventually results in marked impairment in overall functioning and health‐related quality of life. A worldwide prevalence rate of 2.4% has been reported. The risk of suicide is higher in people with bipolar disorder than those with other mental disorders. Therefore, effective management of bipolar disorder in the maintenance period is warranted to minimize the risk of relapse or recurrence. Although lithium has been the standard treatment of bipolar disorder for many years, it is associated with adverse effects and teratogenicity. Lamotrigine is approved to be expected for prevention of recurrence for the maintenance treatment of bipolar disorder. In addition, lamotrigine is as effective as lithium. Therefore, we performed a systematic review to confirm the efficacy and safety of lamotrigine in the maintenance treatment of bipolar disorder.[1]
Our review included 11 studies involving 2314 participants. We found low‐certainty evidence supporting the use of lamotrigine over placebo for people with bipolar disorder. Lamotrigine was found to be more effective than placebo for minimizing recurrence of bipolar depression at one year. Moderate‐certainty evidence indicated that a similar safety profile compared to placebo. Treatment withdrawal at 6 to 12 months was more frequent amongst participants in the placebo groups when compared with the lamotrigine groups. Compared to lithium, we found low‐certainty evidence indicating that it was comparable to lithium in the outcomes of bipolar disorder symptoms except for recurrence of bipolar mania. Current evidence also found that lamotrigine increased incidence of exacerbated bipolar manic symptoms when compared to lithium. In addition, adverse events experienced by participants treated with lamotrigine were lower than those reported in the lithium groups.
In this review we attempted to ensure the highest possible level of certainty of the evidence, we excluded non‐randomized studies or randomized controlled trials (RCTs) that did not use a standard diagnostic process. Our review findings showed that lamotrigine was more effective than placebo on recurrence of bipolar disorder, and the incidence of adverse events was comparable between groups. Furthermore, lamotrigine was found to be more tolerable than lithium, which has been the standard treatment approach in clinical practice. The key to treatment during the maintenance phase of bipolar disorder is continuity of treatment. Considering these facts, we would like to highlight that lamotrigine poses as a viable treatment option.
Pharmacological Properties and Clinical Efficacy in Epilepsy
Lamotrigine is an antiepileptic drug which is believed to suppress seizures by inhibiting the release of excitatory neurotransmitters. Efficacy has been demonstrated for lamotrigine as add-on therapy to existing regimens in patients with resistant partial seizures. Total seizure frequency was reduced by 17 to 59% compared with placebo, and 13 to 67% of patients experienced reductions of ⩾50% in seizure frequency. Secondarily generalised tonic-clonic seizures respond well to lamotrigine, and there is preliminary evidence of improvement in patients with primary generalised seizures, Lennox-Gastaut syndrome and in children with multiple seizure types. Seizure control has been maintained in patients who have continued to receive lamotrigine as monotherapy after discontinuation of other medications. Results of one trial suggest similar efficacy for lamotrigine monotherapy as for carbamazepine, but confirmation of its use in this setting awaits more extensive controlled comparisons with established agents. Lamotrigine is an antiepileptic drug which is believed to suppress seizures by inhibiting the release of excitatory neurotransmitters. Efficacy has been demonstrated for it as add-on therapy to existing regimens in patients with resistant partial seizures. Total seizure frequency was reduced by 17 to 59% compared with placebo, and 13 to 67% of patients experienced reductions of ⩾50% in seizure frequency. Secondarily generalised tonic-clonic seizures respond well to lamotrigine, and there is preliminary evidence of improvement in patients with primary generalised seizures, Lennox-Gastaut syndrome and in children with multiple seizure types. Seizure control has been maintained in patients who have continued to receive lamotrigine as monotherapy after discontinuation of other medications. Results of one trial suggest similar efficacy for lamotrigine monotherapy as for carbamazepine, but confirmation of its use in this setting awaits more extensive controlled comparisons with established agents.[2]
Lamotrigine probably exerts its anticonvulsant effects by blocking voltage-dependent sodium channels, thus stabilising the presynaptic membrane and preventing the release of excitatory neurotransmitters, predominantly glutamate. Its lack of activity at the N-methyl-d-aspartate (NMDA) receptor negates the possibility of phencyclidine-like central nervous system (CNS) effects occurring with its use.The profile of anticonvulsant activity of lamotrigine in animal studies resembles that of phenytoin and carbamazepine. Lamotrigine suppressed seizures induced in rodents by maximal electroshock and pentetrazol (pentylenetetrazol), suggesting activity against partial and generalised tonic-clonic seizures. The drug also decreased electrically evoked after-discharge duration in various animals, a further indication of activity in simple and complex partial seizures. Activity was demonstrated in the photically evoked after-discharge test, which is considered a model of the absence seizure, and some patients with this seizure type have responded to lamotrigine (see Clinical Efficacy).Single oral doses of lamotrigine 240mg have suppressed interictal epileptiform discharges recorded by electroencephalographic tracings in epileptic patients, but to a lesser extent than diazepam 20mg, and doses of it 120 to 240mg reduced the photosensitivity range in patients with a photoconvulsive response. Effects on psychomotor function have been minimal in healthy volunteers administered single doses of lamotrigine 120 to 300mg, and were fewer than occurred with diazepam 10mg, phenytoin 1000mg or carbamazepine 400 and 600mg.
Linear pharmacokinetics are demonstrable for lamotrigine over the dose range 50 to 400mg, with some recent reports indicating linearity up to 700mg in epileptic patients receiving concomitant enzyme-inducing antiepileptic drugs. Maximum plasma lamotrigine concentrations determined in kinetic studies in healthy subjects and patients with epilepsy were generally achieved within 1 to 3 hours of oral administration of doses in this range. Bioavailability of lamotrigine is calculated as approximately 98%, and apparent volume of distribution as 0.9 to 1.3 L/kg. Plasma protein binding is about 55%.Lamotrigine undergoes biotransformation by glucuronidation to a 2-N-glucuronide derivative, which accounts for approximately 75 to 90% of the amount recoverable in the urine after a single oral dose, and to a 5-N-glucuronide. Under steady-state conditions, 43 to 87% of an oral dose of lamotrigine was retrievable within 24 hours, 95% as the glucuronide metabolite and the remainder as parent drug. Total clearance is about 0.03 L/h/kg in healthy persons.The steady-state elimination half-life (t½) of lamotrigine in healthy young adults is approximately 25 to 30 hours. This value is halved in epileptic patients also receiving enzyme-inducing drugs such as carbamazepine and phenytoin, and doubled in the presence of valproic acid. Despite findings that the t½ of it was lengthened in patients with Gilbert’s syndrome (asymptomatic unconjugated hyperbilirubinaemia), and in the elderly, t½ values nonetheless remained within the normal range. The pharmacokinetic profile of lamotrigine in children has not been well described, but elimination of the drug appears faster in these patients than in adults.Any relationship between plasma lamotrigine concentrations and therapeutic effect appears tenuous; therefore, dosage should be modified according to clinical response rather than to a defined therapeutic range.
References
[1]Hashimoto Y, Kotake K, Watanabe N, Fujiwara T, Sakamoto S. Lamotrigine in the maintenance treatment of bipolar disorder. Cochrane Database Syst Rev. 2021 Sep 15;9(9):CD013575.
[2]Goa, K L et al. “Lamotrigine. A review of its pharmacological properties and clinical efficacy in epilepsy.” Drugs vol. 46,1 (1993): 152-76.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringRepaglinide is commonly used to help lower blood sugar levels in people with type 2 diabetes and can be used for other conditions as determined.....
May 8,2025APILamotrigine
84057-84-1You may like
- Lamotrigine
-
- $46.00 / 500mg
- 2025-12-17
- CAS:84057-84-1
- Min. Order:
- Purity: 99.95%
- Supply Ability: 10g
- Lamotrigine
-
- $46.00 / 500mg
- 2025-12-17
- CAS:84057-84-1
- Min. Order:
- Purity: 99.95%
- Supply Ability: 10g
- Lamotrigine
-

- $980.00/ kg
- 2025-12-16
- CAS:84057-84-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000