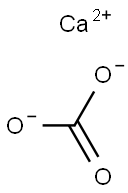
Lewis Structure of Calcium Carbonate and the Reaction with HCl
May 7,2025
Calcium carbonate is a widespread compound found in chalk, lime, marble, and more. This substance is a crucial pillar of human life – it is used in construction, to manufacture paper and plastic, and in many other spheres. It is also popular in the food industry as a natural white colorant.
Lewis Structure
The Lewis structures for Calcium Carbonate (CaCO3) consist of a Calcium ion (Ca^(2+)) bonded to a carbonate ion (CO3^(2-)). The carbonate ion exhibits three resonance structures with one double bond and one single bond between Carbon and Oxygen atoms, and formal charges of -1 on two Oxygen atoms.

Calcium carbonate is a solid white substance that won't dissolve in it completely: the water will turn a muddy color and a white precipitate will appear. But if the reaction with water takes place in the presence of carbon dioxide, it yields calcium hydrogen carbonate, a soluble acidic salt.
Reaction with HCl
Hydrochloric acid is a strong monobasic acid and is obtained via the dissolution of hydrogen chloride HCl in water. It is a colorless liquid, although industrial acid can have a yellow tint, often due to a mixture of iron. The properties of this solution depend directly on the concentration of hydrogen chloride. The salts of hydrochloric acid are called chlorides.
This substance is very caustic and requires careful handling: even a small drop on the skin will cause a severe chemical burn. When working with strong acids, you should always have neutralizers at hand – weak alkaline solutions, sodium bicarbonate (baking soda), etc.
When calcium carbonate (CaCO3) reacts with hydrochloric acid (HCl), itproduces calcium chloride (CaCl2), carbon dioxide (CO2), and water (H2O).
- Related articles
- Related Qustion
- Benefits and side effects of Calcium carbonate supplements Dec 26, 2024
Calcium carbonate is a nutritional supplement used to supplement calcium. Calcium is an essential nutrient for normal growth and development.
- Is daily consumption of calcium carbonate considered safe? Aug 26, 2024
Calcium carbonate is generally safe for daily calcium supplementation.
- Is calcium carbonate easily soluble in water? What are its applications? Mar 21, 2024
Calcium carbonate is a common substance on Earth, found in minerals like aragonite, calcite, chalk, and travertine, as well as being a major constituent of certain animal bones and shells.
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringThe Lewis structure of KBr is composed of a potassium positive (K+) ion and a bromine negative ion (Br+), where the bromine ion carries 4 lone pairs of electrons, and the two ions are surrounded by "[ ]".....
May 7,2025APICalcium carbonate
471-34-1You may like
Calcium carbonate manufacturers
- Calcium carbonate
-
- 2025-12-06
- CAS:471-34-1
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Calcium carbonate
-

- $0.00 / 1kg
- 2025-12-05
- CAS:471-34-1
- Min. Order: 1kg
- Purity: 99.51%
- Supply Ability: 1000kg
- Calcium carbonate
-

- $0.00 / 1kg
- 2025-12-05
- CAS:471-34-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20MT