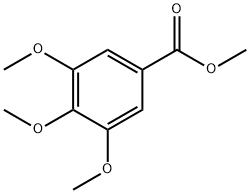
Methyl 3,4,5-Trimethoxybenzoate: A Versatile Molecule as Membrane-Active Anticancer Agents
Sep 30,2025
Methyl 3,4,5-trimethoxybenzoate exhibits specific forms and characteristics. It appears as a white to greyish-white crystalline powder with a density of 1.134 g/cm³. Its melting point ranges between 82–84°C, boiling point between 274–275°C, and flash point at 115.2°C. At 25°C, its vapour pressure is 0.00538 mmHg. These physical parameters provide crucial guidance for the compound's storage, transportation, and subsequent processing applications. Methyl 3,4,5-trimethoxybenzoate possesses excellent reactivity due to functional groups including methyl, methoxy, and methyl ester. It can undergo various chemical reactions such as esterification and methylation. Through reactions with other compounds, it can yield products with specific functionalities, laying the foundation for its application in chemical synthesis. Regarding its applications, Methyl 3,4,5-trimethoxybenzoate is primarily employed as a pharmaceutical intermediate. It serves as a key raw material in the production of drugs such as the anxiolytic trimetazidine and the gastrointestinal medication trimebutine maleate. Additionally, it is utilised in the manufacture of antimicrobial synergists like methoxybenzamidine, thereby providing substantial support to the advancement of the pharmaceutical industry.

Methyl 3,4,5-trimethoxybenzoate in Catechin in Anionic Phospholipid Membranes
There is compelling evidence from biochemical and biological studies that green tea catechins can produce diverse beneficial effects on cancer in humans, including prevention of cancer, synergistic anticancer effect, and inhibition of metastasis. Catechins display many anti-carcinogenic and anti-mutagenic promising protective effects in cancer, including breast, esophagus, prostate, stomach, small intestine, colon, liver, and lung. In spite of the exceptional anticancer characteristic of catechins, they present an important restraint: their poor bioavailability, which is associated to their low stability in neutral of slightly alkaline solutions and their inefficiency to conveniently traverse cellular membranes. There are extensive efforts to develop new catechin-derived compounds and improve such bioavailability issue. An important catechin derivative is Methyl 3,4,5-trimethoxybenzoate of catechin, which has been established to display a high antiproliferative activity against malignant melanoma cells.[1]
The amphiphilic nature of Methyl 3,4,5-trimethoxybenzoate of catechin indicates that the membrane could be a potential site of its action, therefore, the study on the influence of this semisynthetic catechin on the lipid component of membranes is crucial in order to get insight into the mechanism of anticarcinogenic action of this compound. The examination of this TMBC-membrane interaction could also shed light on the mechanisms underlying other demonstrated beneficial effects of catechins. It has been shown that Methyl 3,4,5-trimethoxybenzoate of catechin is incorporated into membranes composed of the most abundant phospholipids in eukaryotic membranes phosphatidylcholine and phosphatidylethanolamine, where it perturbed the structural properties of these phospholipid bilayers. The objective of this work was to characterize the molecular interactions of the anticarcinogenic semisynthetic catechin derivative Methyl 3,4,5-trimethoxybenzoate of catechin with anionic phospholipids membranes formed by DMPS. Our DSC data supported that TMBC is able to incorporate into DMPS bilayers and to embed between the molecules of phospholipids, where it can reduce the cooperativity and lower the transition temperature of the gel to liquid-crystalline phase transition.
The presence of multiple peaks in the thermograms suggested that different TMBC-phospholipids domains were formed. X-ray diffraction measurements indicated that TMBC was able to partially induce the formation of interdigitated gel phase in DMPS, and in the liquid crystalline phase, the presence of Methyl 3,4,5-trimethoxybenzoate of catechin produced a decrease of the bilayer thickness. The position of TMBC in the bilayer allowed the formation of new hydrogen bonds between the carbonyl groups of the phospholipid and the hydroxyl groups of the catechin, and also granted the catechin to disturb the intermolecular hydrogen bonding between neighboring DMPS molecules. Taken together, all these results point to a strong interaction between Methyl 3,4,5-trimethoxybenzoate of catechin and anionic bilayers generating physical perturbations, which could modify membrane function.
References
[1]Aranda E, Teruel JA, Ortiz A, Pérez-Cárceles MD, Rodríguez-López JN, Aranda FJ. 3,4,5-Trimethoxybenzoate of Catechin, an Anticarcinogenic Semisynthetic Catechin, Modulates the Physical Properties of Anionic Phospholipid Membranes. Molecules. 2022 May 3;27(9):2910. doi: 10.3390/molecules27092910. PMID: 35566261; PMCID: PMC9105813.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringL-rhamnose is ubiquitous in many natural products, glycoproteins, and structural polysaccharides, predominantly existing in L-Rhamnose monohydrate.....
Sep 30,2025SaccharidesMethyl 3,4,5-trimethoxybenzoate
1916-07-0You may like
Methyl 3,4,5-trimethoxybenzoate manufacturers
- Methyl 3,4,5-trimethoxybenzoate
-

- $5.00 / 25kg
- 2025-11-27
- CAS:1916-07-0
- Min. Order: 1kg
- Purity: ≥99%
- Supply Ability: 200mt/year
- Methyl 3,4,5-trimethoxybenzoate
-
- $37.00 / 500mg
- 2025-11-04
- CAS:1916-07-0
- Min. Order:
- Purity: 99.87%
- Supply Ability: 10g
- Methyl 3,4,5-trimethoxybenzoate
-
- $37.00 / 500mg
- 2025-11-04
- CAS:1916-07-0
- Min. Order:
- Purity: 99.87%
- Supply Ability: 10g