N-(2-hydroxyethyl)-N-methyl-4-toluidine: Synthesis and Application
Dec 7,2022
General description
N-(2-hydroxyethyl)-N-methyl-4-toluidine is an important organic synthesis intermediate and functional additive for chemical materials.
Synthetic routes
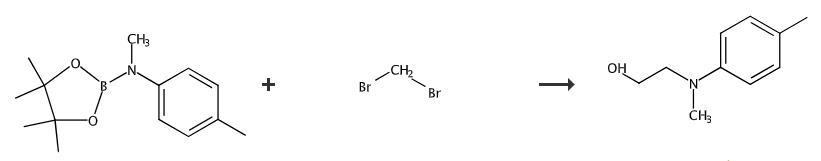
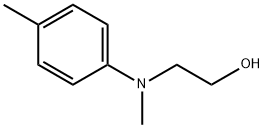
Fig. 1 The synthetic method 1 of N-(2-hydroxyethyl)-N-methyl-4-toluidine.
Add reactant (0.2 mmol, 1.0 equiv), THF (2.0 mL) and CH2Br2 (0.6 mmol, 3.0 equiv) to a flame-dried 10 mL Schlenk tube in a glove box. Seal and take out of the glove box. Cool the reaction mixture to -78°C. Add nBuLi (0.56 mmol, 2.8 equiv) dropwise under N2 atmosphere within 3minutes. Stir the reaction at -78°C for 30 minutes and add ZnCl2 (0.1 mL, 0.5equiv, 1.0 M in Et2O). Allow the mixture to warm to room temperature and stir for 1 hour. Cool the mixture to 0°C. Add a premixture of H2O2 (30% in H2O, 0.5 mL) and NaOH (2.0 M, 1.0 mL). Stir the mixture at room temperature for another 1 hour and dilute with water (20 mL). Extract with DCM (30 mL x 2) and dry over Na2SO4. Filter and concentrate under vacuum. Purify the crude product by silica gel flash column chromatography to obtain product. 1H NMR (CDCl3, 500 MHz) δ 7.08 (d, J = 8.4 Hz, 2H), 6.78 (d, J = 8.4 Hz,2H), 3.80 (t, J = 5.6 Hz, 2H), 3.43 (t, J = 5.4 Hz, 2H), 2.93 (s, 3H), 2.28 (s, 3H), 2.01 (brs, 1H). 13C NMR (CDCl3, 125 MHz) δ 148.3, 129.8, 127.1, 114.0, 60.1, 56.2, 39.1, 20.4 [1].
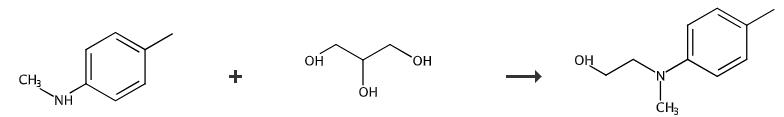
Fig. 2 The synthetic method 2 of N-(2-hydroxyethyl)-N-methyl-4-toluidine.
Add [Ru(p-cymene)Cl2]2 (1.5 mg, 0.5 mol%) and 1,1'-bis(di-cyclohexylphosphino)-ferrocene (3.0 mg, 1.05 mol%) to a 4 mL vial in an nitrogen-filled glove box followed by 0.25 mL 1,4-dioxane. Seal the vial. Stir the reaction mixture at 110 °C for 1 hour outside the glove box. Allow the reaction mixture to cool down to room temperature. Add N-methyl-(2-thienylmethyl)amine (0.25 mL of 0.024 M stock solution, 1.2 mol%) into the above mixture inside glove box. Seal the vial. Stir the resulted mixture outside glove box at 110 °C for 0.5 hour to generate the catalyst in situ. Add glycerol (138.1 mg), KOtBu (2.8 mg, 5 mol%), the in situ generated catalyst, 1,4-dioxane (0.5 mL) to a 10 mL glass tube in the glove box followed by N-alkyl-aniline (0.5 mmol). Seal the tube with a screw cap, 2 mm stabilizing PTFE disc and a 2 mm thick PTFE-lined silicone disc. Transfer the mixture outside glove box. Stir the reaction mixture at 150 °C for 20 hours. Allow the reaction mixture to cool to room temperature. Concentrate the mixture in vacuum. Purify the crude product by flash chromatography (eluent: 20% ethyl acetate in petroleum ether). 1H NMR (400 MHz, CDCl3): δ (ppm) 7.06 (d, J = 8.3 Hz, 2H), 6.76 (d, J = 8.4 Hz, 2H), 3.79 (t, J = 5.5 Hz, 2H), 3.41 (t, J = 5.5 Hz, 2H), 2.92 (s, 3H), 2.27 (s, 3H), 1.94 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) 148.3, 129.6 (2C), 127.0, 113.9 (2C), 60.0, 56.1, 39.0, 20.3 [2].
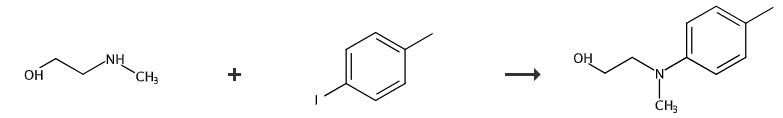
Fig. 3 The synthetic method 3 of N-(2-hydroxyethyl)-N-methyl-4-toluidine.
Place 4-iodotoluene (2.18 g), copper(I) iodide (48.0 mg, 0.250 mmol) and sodium hydroxide (0.800 g, 20.0 mmol) in a sealable tube. Flush the reaction mixture with argon. Add 2-(methylamino)ethanol (8.03 mL, 100 mmol) to the reaction mixture. Seal the tube. Heat the reaction mixture at 90 °C for 19 hours. Cool the reaction mixture to room temperature. Partition the reaction mixture between water and CH2Cl2 (3 times). Wash the combined organics with 0.5 M NaOH and saturated brine. Dry the combined organics over MgSO4. Filter the combined organics. Concentrate the combined organics in vacuo. Purify the crude product by flash chromatography on silica gel, eluting with EtOAc/Petrol (1:9 to 1:4). 1H NMR (400 MHz; CDCl3): δ 7.09-7.06 (m, 2 H), 6.76 (d, J= 8.4 Hz, 2 H), 3.79 (t, J= 5.4 Hz, 2 H), 3.42 (t, J= 5.4 Hz, 2 H), 2.92 (s, 3 H), 2.81 (s, 3 H). 13C NMR (100 MHz; CDCl3): δ 148.2 (C), 129.7 (CH), 126.8 (C), 113.7 (CH), 59.9 (CH2), 56.0 (CH2), 38.9 (Me), 20.2 (Me) [3].
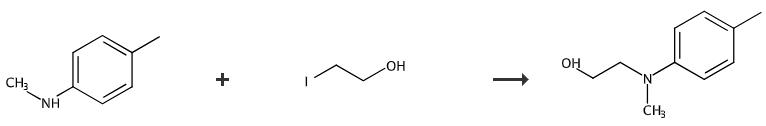
Fig. 4 The synthetic method 4 of N-(2-hydroxyethyl)-N-methyl-4-toluidine.
Add 60% Nail (dispersed in mineral oil, 279.5 mg, 6.99 mml) to a solution of methyl-p-tolylamine (6.99 mmol) in DMF (30 mL) at room temperature with stirring under nitrogen. Stir the mixture for 1 hour. Add 2-iodoethanol (0.654 mL, 8.39 mmol) dropwise to the mixture. Stir the mixture at 60 °C for 48 hours. Extract the reaction mixture with ethyl acetate. Wash the combined organic extract with brine. Dry the combined organic extract over anhydrous magnesium sulfate. Filter the combined organic extract. Concentrate the combined organic extract under reduced pressure. Purify the residue by flash column chromatography (eluent, n-hexane/EtOAc = 3:1). 1H NMR 400 MHz, CDCl3 δ 7.04 (m, 2H), 6.74 (m, 2H), 3.76 (t, 2H, J= 5.2 Hz), 3.39 (t, 2H, J= 5.2 Hz), 2.89 (s, 3H), 2.25 (s, 3H). 13C NMR 100 MHz, CDCl3 δ 148.1, 129.7, 127.0, 113.8, 59.9, 56.1, 39.0, 20.2 [4].
Application
As an intermediate in organic synthesis
N-(2-hydroxyethyl)-N-methyl-4-toluidine is an important synthetic intermediate for Oxindoles, Thio-oxindoles, 3,4-Dihydro-1 H-quinolin-2-ones, and 1,2,3,4-Tetrahydroquinolines. A copper(II)-catalyzed approach to oxindole derivs., thioxindole derivs., 3,4-dihydro-1H-quinolinone derivs. and 1,2,3,4-tetrahydroquinoline derivs. by a formal C-H, Ar-H coupling is described. In a new variant, 2-ethylhexanoic acid copper(2+) salt (2:1) [i.e., copper(II) 2-ethylhexanoate] has been identified as an inexpensive and efficient catalyst for this transformation, which utilizes atm. oxygen as the re-oxidant. The synthesis of the target compds. as achieved using starting materials, such as 2-methyl-3-(methylphenylamino)-3-(oxo)propanoic acid Et ester, 2-cyano-N-methyl-N-(phenyl)propanamide, P-[1-methyl-2-(methylphenylamino)-2-oxoethyl]phosphonic acid ester and related substances. The title compds. thus formed included (oxo)indolecarboxylic acid esters, (thioxo)indolecarboxylic acid esters, tetrahydroquinoline derivs., benzo[1,2-b:4,5-b']dipyrrole, pyrido[2,3-g]quinoline, pyrrolo[2,3-g]quinoline derivs. Quinoline derivs. included 2,3-dihydro-1-methyl-2-oxo-4,4(1H)-quinolinedicarboxylic acid 4,4-di-Et ester and related substances [3].
References
[1] Xie Q, Dong G. Aza-Matteson Reactions via Controlled Mono-and Double-Methylene Insertions into Nitrogen–Boron Bonds[J]. Journal of the American Chemical Society, 2021, 143(36): 14422-14427.
[2] Xin Z, Jia L, Huang Y, et al. Ru‐Catalyzed Switchable N‐Hydroxyethylation and N‐Acetonylation with Crude Glycerol[J]. ChemSusChem, 2020, 13(8): 2007-2011.
- Related articles
- Related Qustion
- N-(2-hydroxyethyl)-N-methyl-4-toluidine: Overview, Applications as Catalyst and its Preparation Method Nov 27, 2024
N-(2-hydroxyethyl)-N-methyl-4-toluidine serves as a catalyst with significant applications in the formulation of unsaturated polyester resin putty compositions for powder coating technology.
9, 9-Bis (methoxymethyl) fluorene can be used as an intermediate in the fields of materials or drug molecules through polymerization reactions or functional groups.....
Dec 7,2022Organic Synthesis IntermediateCarrageenan is an additive used to thicken, emulsify, and preserve foods and drinks.....
Dec 7,2022Food AdditivesN-(2-HYDROXYETHYL)-N-METHYL-4-TOLUIDINE
2842-44-6You may like
N-(2-HYDROXYETHYL)-N-METHYL-4-TOLUIDINE manufacturers
- N-Methyl-N-Hydroxyethyl-P-Toluidine (MHPT)
-

- $55.00/ kg
- 2025-12-04
- CAS:2842-44-6
- Min. Order: 1000kg
- Purity: 99.0%
- Supply Ability: 100 tons
- N-(2-HYDROXYETHYL)-N-METHYL-4-TOLUIDINE
-
- $10.00 / 1KG
- 2025-12-03
- CAS:2842-44-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt