Nafcillin: Antimicrobial Activity, Susceptibility, Administration and Dosage, Clinical Uses etc.
Mar 15,2022
Nafcillin, 6-(2-ethoxy-1-naphthamido) penicillanic acid, is a semisynthetic penicillin derived from the penicillin nucleus, 6-aminopenicillanic acid. Similar to methicillin and the isoxazolyl penicillins, it is resistant to staphylococcal penicillinase and has a predominately Gram-positive spectrum. Developed in 1961, it has since been widely used in the United States and a small number of other countries for the parenteral treatment of serious b-lactamase-producing staphylococcal infections.
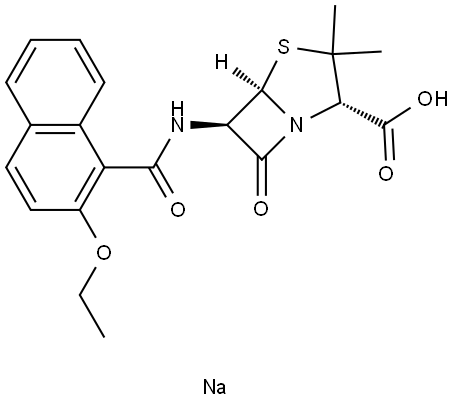
Nafcillin comes as a 1- and 2-g parenteral formulation for intravenous and intramuscular use. Its molecular formula is C21H22N2O5S·H2O, and a molecular weight of 454.5. The chemical structure is shown in Figure 6.1. It acts like other penicillins in the penicillin-binding proteins, principally PBPs 1a, 1b, and 2.
ANTIMICROBIAL ACTIVITY
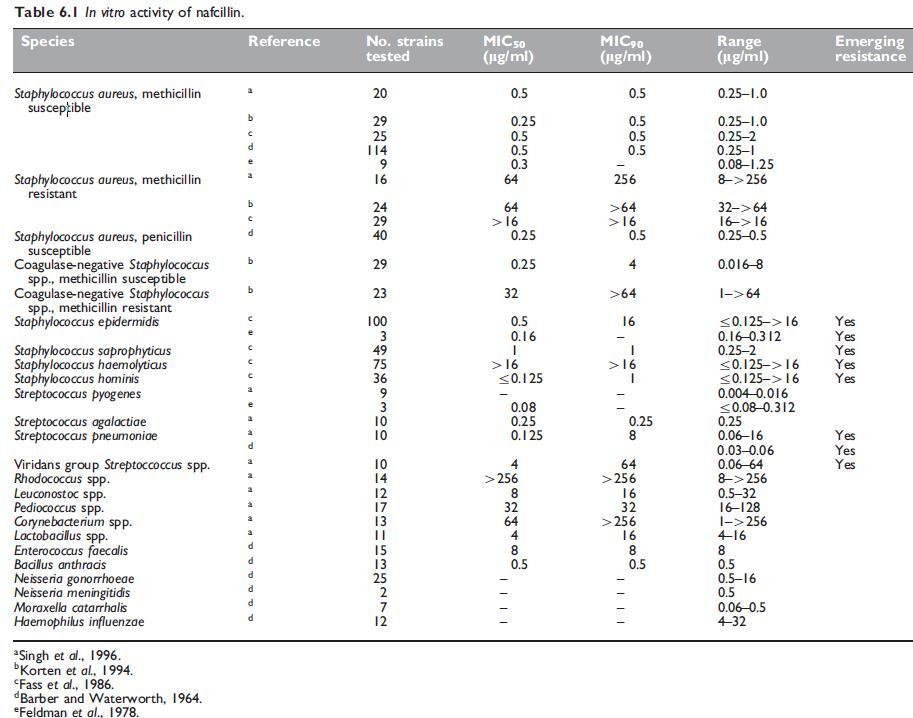
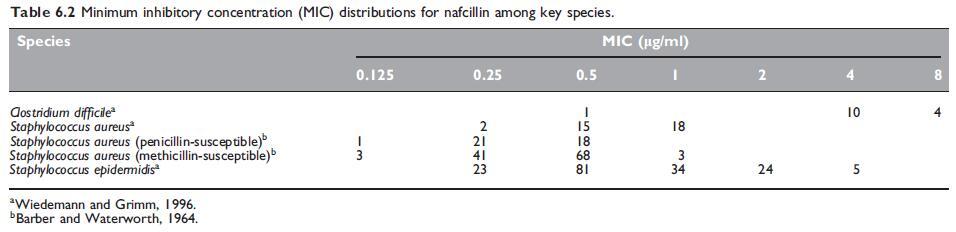
The in vitro susceptibility of key pathogens to nafcillin are summarized in Tables 6.1 and 6.2.
a. Routine susceptibility
Nafcillin has a very similar antibacterial spectrum to the isoxazolyl penicillins (see Chapter 5, Isoxazolyl Penicillins: Oxacillin, Cloxacillin, Dicloxacillin and Flucloxacillin). It is about as active as oxacillin against both penicillin G-susceptible and penicillin G-resistant Staphylococcus aureus strains (Klein et al., 1963). Stability of nafcillin in the presence of staphylococcal b-lactamase (penicillinase) is similar to that of methicillin and greater than that of the isoxazolyl penicillins. Nafcillin shows superior activity to glycopeptides in an animal model of subcutaneous abscesses induced by methicillin-susceptible S. aureus (Wood and Wisniewski, 1994). Methicillin-resistant S. aureus strains are resistant to all penicillinase-resistant penicillins, including nafcillin (Barber and Waterworth, 1964; Richmond et al., 1977).
b. Emerging resistance and cross-resistance
In common with isoxazolyl penicillins, strains of S. aureus harboring the mecA gene encoding PBP2a are resistant to nafcillin, as well as methicillin. Nafcillin susceptibility is also reduced in the setting of altered penicillin-binding proteins in Streptococcus pneumoniae which confer reduced susceptibility or resistance to penicillin G, other penicillins, and cephalosporins. In both regards, nafcillin resistance can be inferred from resistance to methicillin or oxacillin or the presence of mecA in S. aureus and coagulase-negative staphylococci, and by the presence of oxacillin resistance in S. pneumoniae. Presumably, a situation analogous to that of S. pneumoniae applies to the viridans group of streptococci.
MECHANISM OF DRUG ACTION
Nafcillin, like other penicillins (see Chapter 1, Benzylpenicillin (Penicillin G)), inhibits peptidoglycan synthesis by inhibiting the transpeptidase enzymes PBP1a, 1b, and 2.
MODE OF DRUG ADMINISTRATION AND DOSAGE
a. Adults
Owing to low and unpredictable absorption, nafcillin is not recommended for oral administration. Nafcillin can be given either i.m. or i.v. Intramuscular administration results in a peak concentration of 5–8 mg/l after 500-mg to 1-g doses (Martindale, 2007). The usual adult i.v. dosage is 1 g 4-hourly, but this can be doubled for the treatment of severe infections, in particular endocarditis. Doses of up to 18 g i.v. daily have been given to adults with no ill effects. The drug should be given i.v. using techniques as described for penicillin G (see Chapter 1, Benzylpenicillin (Penicillin G)).
b. Newborn infants and children
The usual parenteral pediatric dose is 25 mg/kg 4-hourly; higher doses of 50 mg/kg 4-hourly can be given safely. In newborns, the recommended dosage for severe infection is 100 mg/kg/day, given in two divided doses for infants less than 7 days of age, and in three divided doses for those older than 7 days (Banner et al., 1980). In those with birth weights less than 2000 g, unnecessarily high serum levels are obtained with these doses. A dose of 20 mg/kg body weight, administered 8-hourly, is probably sufficient for those with low birth weights (Banner et al., 1980).
PHARMACOKINETICS AND PHARMACODYNAMICS
a. Bioavailability
Nafcillin is comparatively poorly and inconsistently absorbed from the gastrointestinal tract compared with the isoxazolyl penicillins (Watanakunakorn, 1977). Doses of 500mg and 1 g orally yield peak concentrations of 3.271.9mg/l and 7.772.7mg/l, respectively; thus, serum levels following oral nafcillin are low and irregular. Administration with food halves absorption (Klein et al., 1963; Whitehouse et al., 1963; Watanakunakorn, 1977). Therefore, oral administration of nafcillin is not recommended (Klein et al., 1963; Watanakunakorn, 1977).
b. Drug distribution
Following i.v. infusion of a 0.5-g dose of nafcillin over 15 minutes to adults, the serum level is 11 mg/ml at the end of the infusion and 0.5 mg/ml at 6 hours (Neu, 1982). After an i.m. injection of 1-g nafcillin, a peak serum level of about 8 mg/ml is reached 1 hour later; it falls to about 0.5 mg/ml at 6 hours (Whitehouse et al., 1963).
Concomitant oral administration of probenecid increases and prolongs nafcillin levels, similar to other penicillins (Klein et al., 1963). Probenecid reduces urinary recovery by 50%, decreases both renal and nonrenal clearance, and doubles the area under the concentration– time curve (Waller et al., 1982).
c. Clinically important pharmacokinetic and pharmacodynamic features
Like other penicillins, the antistaphylococcal penicillins show only slight concentration-dependent killing, with maximum effects reached at concentrations 3- to 4-fold higher than the MIC (Craig and Ebert, 1991). The in vitro postantibiotic effect of antistaphylococcal penicillins against S. aureus is moderate at most, of the order of 1.5– 2 hours depending on concentration and time of exposure (Craig and Gudmundsson, 1996). In vivo, the postantibiotic effect is somewhat longer, for example 3 hours for nafcillin against S. aureus (Craig and Gudmundsson, 1996).
d. Excretion
About 30% of an i.m. administered dose of nafcillin can be recovered from the urine where concentrations reach as high as 1000 mg/ml. A considerably smaller amount of active nafcillin (about 19% of the administered dose) is recovered from the urine after i.m. administration if it is given with probenecid (Klein et al., 1963; Waller et al., 1982).
e. Drug interactions
Nafcillin has few drug interactions. There are conflicting data about the potential for interaction with cyclosporin, either decreasing plasma levels (Veremis et al., 1987) or increasing its nephrotoxicity without altering plasma levels (Jahansouz et al., 1993). More important is the interaction with warfarin. Nafcillin appears to be an inducer of warfarin metabolism, and warfarin requirements are likely to at least double while the patient is receiving nafcillin (Qureshi et al., 1984; Fraser et al., 1989; Davis et al., 1991; Kim et al., 2007).
TOXICITY
Overall adverse reaction rates to nafcillin when used as definitive treatment are approximately 20–30% (Kancir et al., 1978; Maraqa et al., 2002). Different rates, either higher or lower, have been recorded when used for prolonged periods, such as for outpatient treatment (Dahlgren, 1997; Wynn et al., 2005). Serious reaction rates are o5%.
a. Hypersensitivity reactions
Nafcillin, like other penicillins, may cause the same hypersensitivity reactions that occur with penicillin G (see Chapter 1, Benzylpenicillin (Penicillin G)). Most common is skin rash (Dahlgren, 1997). The drug is contraindicated in any patient with a history of penicillin sensitivity. Rash is less likely with nafcillin than with oxacillin (Maraqa et al., 2002).
b. Nephrotoxicity
Parry et al. (1973) described a patient who developed renal damage due to methicillin, which resolved when lincomycin was substituted. Later, when therapy was changed to nafcillin, the hypersensitivity nephritis recurred. Nephropathy has been reported on many occasions with methicillin, but less commonly with other penicillinase-resistant penicillins. If nephropathy develops after the use of one penicillin analog, it is likely to recur if any other penicillin is subsequently used.
c. Hypokalemia
Nafcillin administered in large doses i.v. (200 mg/kg/day) can cause hypokalemia and associated alkalosis (Mohr et al., 1979). Nafcillin acts as a nonreabsorbable anion and increases passive renal distal tubular potassium excretion. This is similar to what occurs with other penicillins used in large doses, such as penicillin G. Hypokalemia may resolve when the nafcillin dose is reduced (Andreoli et al., 1980).
d. False-positive tests
Nafcillin in the urine can cause a false-positive urine reaction for protein when the sulfasalicylic test is used, but not with the dipstick test. Unrecognized, this may lead to unnecessary cessation of the drug and even renal biopsy (Line et al., 1976). Penicillin G and oxacillin can also cause false-positive urine protein determinations, but to a lesser degree.
e. Hematologic side-effects
Neutropenia with concomitant fever occurred in one patient receiving a daily dose of 12 g i.v. nafcillin. This complication resolved when the drug was stopped (Sandberg et al., 1975). In another patient, i.v. nafcillin therapy (12 g daily) was associated with the development of agranulocytosis, which only improved after the drug was discontinued (Markowitz et al., 1975). Greene and Cohen (1978) described neutropenia in three children receiving i.v. nafcillin, which also resolved when nafcillin was ceased.
f. Skin and tissue necrosis
This can occur after accidental subcutaneous extravasation of i.v. nafcillin, and may necessitate multiple tissue debridements and skin grafting. In animals, tissue necrosis occurs after subcutaneous inoculation of nafcillin, but not with oxacillin, methicillin, and cephalothin (Tilden et al., 1980). In humans, nafcillin-induced tissue injury can be prevented by prompt administration of hyaluronidase into the site of extravasation (Zenk et al., 1981).
g. Hepatotoxicity
Hepatotoxicity has been described with all the antistaphylococcal penicillins. Rates of toxicity, at least in the setting of outpatient intravenous therapy, are significantly lower for nafcillin than oxacillin (Maraqa et al., 2002) (see Chapter 5, Isoxazolyl Penicillins: Oxacillin, Cloxacillin, Dicloxacillin and Flucloxacillin).
h. Risks in pregnancy and fetal toxicity
Nafcillin appears to pose no specific risk in pregnancy to either the mother or the fetus, and is generally given category B status.
CLINICAL USES
a. S. aureus infections
Parenteral nafcillin has been used successfully for the treatment of severe S. aureus infections, such as septicemia, endocarditis, osteomyelitis, septic arthritis, pneumonia, meningitis, skin and skin structure infections, and pyomyositis (Eickhoff et al., 1965; Goldenberg and Cohen 1976; Parker and Fossieck, 1980; Carney et al., 1982; Watanakunakorn, 1987; Kim et al., 1989; Daly et al., 1990; Walling and Kaelin, 1991; Givner and Kaplan, 1993). In the USA, nafcillin is widely regarded as the preferred drug for treatment of S. aureus endocarditis (Masur et al., 1978; Sande and Scheld, 1980). In many other countries, however, flucloxacillin and cloxacillin are often used instead of nafcillin.
b. Infections due to coagulase-negative staphylococci
Nafcillin can be used to treat severe hospital-acquired infections caused by these organisms, such as prosthetic valve endocarditis, provided the strain is methicillin sensitive. The addition of either gentamicin or rifampicin, or both, to the nafcillin regimen may improve the results of treatment (Sande and Scheld, 1980). If the strain is methicillin resistant, which is often so if the infection is hospital acquired, vancomycin should be used and the addition of either rifampicin or gentamicin, or both, may be of benefit (Karchmer et al., 1983; Caputo et al., 1987).
c. Other uses
Nafcillin has been shown to be effective in a range of Gram-positive infections (mainly S. aureus) in children (Feldman et al., 1978; Kaplan et al., 1982), including cellulitis, septicemia, endocarditis, osteomyelitis, pneumonia, skin and skin structure infections, and meningitis.
References
Alexander DP, Russo ME, Fohrman DE, Rothstein G (1983). Nafcillininduced
platelet dysfunction and bleeding. Antimicrob Agents Chemother
23: 59.
Ambrose PG, Bhavnani SM, Rubino CM et al. (2007). Pharmacokineticspharmacodynamics
of antimicrobial therapy: it’s not just for mice anymore.
Clin Infect Dis 44: 79.
Andreoli SP, Kleiman MB, Glick MR, Bergstein JM (1980). Nafcillin, pseudoproteinuria
and hypokalemic alkalosis. J Pediatr 97: 841.
Archer GL, Armstrong BC (1983). Alteration of staphylococcal flora in cardiac
surgery patients receiving antibiotic prophylaxis. J Infect Dis 147: 642.
Arthur JD, Bass JW, Keiser JF et al. (1982). Nafcillin-tolerant Staphylococcus
epidermidis endocarditis. JAMA 247: 487.
Banner Jr W, Gooch WM, Burckart G, Korones SB (1980). Pharmacokinetics of
nafcillin in infants with low birth weights. Antimicrob Agents Chemother 17:
691.
Barber M, Waterworth PM (1964). Penicillinase-resistant penicillins and
cephalosporins. Br Med J 2: 344.
Caputo GM, Archer GL, Calderwood SB et al. (1987). Native valve
endocarditis due to coagulase-negative staphylococci. Clinical and
microbiological features. Am J Med 83: 619.
Carney DN, Fossieck Jr BE, Parker RH, Minna JD (1982). Bacteremia due to
Staphylococcus aureus in patients with cancer: report on 45 cases in adults
and review of the literature. Rev Infect Dis 4: 1.
Chambers HF, Miller RT, Newman MD (1988). Right-sided Staphylococcus
aureus endocarditis in intravenous drug abusers: two-week combination
therapy. Ann Intern Med 109: 619.
- Related articles
- Related Qustion
Polyethylene-polypropylene glycol is used as a food additive. It belongs to the family of Epoxides. These are compounds containing a cyclic ether with three ring atoms....
Mar 15,2022Organosilicon compoundsCarbenicillin (disodium alpha-carboxybenzyl-penicillin) is a semisynthetic penicillin derived from the penicillin nucleus, 6-aminopenicillinic acid (6-APA), and can only be administered parenterally (Knudsen et al., 1967).....
Mar 15,2022APINAFCILLIN SODIUM SALT
985-16-0You may like
NAFCILLIN SODIUM SALT manufacturers
- NAFCILLIN SODIUM SALT
-

- $1.00 / 1g
- 2019-12-26
- CAS:985-16-0
- Min. Order: 1g
- Purity: ≥98%
- Supply Ability: g/kg/Ton