Omadacycline: a protein synthesis inhibitor
Dec 19,2023
Description
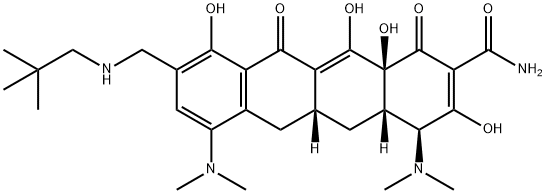
Omadacycline (PTK0796, BAY 73–6944, MK-2764) is available as a mono tosylate salt with a molecular weight of 728.9, and the chemical name (4S,4aS,5aR,12aS)-4,7-bis(dimethylamino)-9-(2,2-dimethyl propyl aminomethyl)-3,10,12,12a-tetrahydroxy-1, 11-dioxo-1, 4, 4a, 5, 5a, 6, 11, 12a- octahydrotetracene-2-carboxamide, 4-methyl benzenesulfonate. A chemical feature that distinguishes omadacycline from tigecycline and eravacycline is the aminomethyl substitution at the C9 position, which provides improved pharmacokinetic parameters, most notably oral bioavailability, lower dose-limiting nausea and vomiting, for which C9 glycylcyclines are infamous, and hindering of efflux-mediated resistance. Discovered and developed by Paratek Pharmaceuticals, omadacycline was licensed to Bayer, Merck, and Novartis throughout its clinical development. Ultimately, the rights were returned to Paratek, who collaborated with Zai Lab (Shanghai) Co., Ltd. to commercialize the drug in China.
Synthetic method
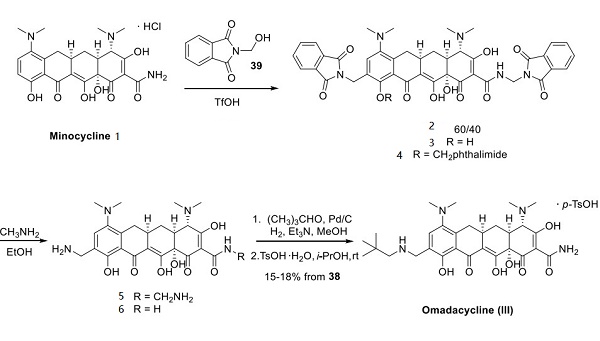
Several syntheses of omadacycline have been published, and the most extensive scale route is described below[1] This route advantageously began with minocycline (1), a tetracyclic antibiotic drug first patented in 1961. Minocycline has condensed with N (hydroxymethyl)phthalimide (2) in the presence of triflic acid to give a mixture of the 9-phthalimidomethyl analogs 3 and 4 in approximately a 60:40 ratio. This mixture was treated with methylamine, which resulted in hydrolysis of the phthalimide to give an unreported distribution of methylamine analogs 5 and 6. This mixture was then treated with pivaldehyde under catalytic hydrogenation conditions to affect the reductive amination at position N-9, followed by concomitant removal of the hemiaminal group on the amide, furnishing omadacycline (III) in 15−18% yield for the overall process after conversion to the tosylate salt.
Uses
Omadacycline is a first-in-class aminomethylcycline with broad-spectrum in vitro activity against Gram-positive aerobes, Gram-negative aerobes, anaerobes, and atypical bacteria. Omadacycline retains antibacterial activity against strains expressing the two most common tetracycline resistance mechanisms, bacterial ribosomal protection proteins and tetracycline-specific efflux pumps. Both oral and intravenous (IV) formulations of omadacycline have been approved by the United States Food and Drug Administration (US FDA) for the treatment of acute bacterial skin and skin structure infections (ABSSSI) and community-acquired bacterial pneumonia (CABP)[2]. Omadacycline has demonstrated in vitro activity against resistant gram-positive pathogens, including methicillin-resistant Staphylococcus aureus (MRSA), penicillin- and macrolide-resistant Streptococcus pneumoniae, vancomycin-resistant Enterococcus (VRE), and Clostridioides difficile, pathogens that have been recognized by the US Centers for Disease Control and Prevention as urgent or serious threats[3].
Mechanisms of action
Like all other tetracyclines, omadacycline is primarily a protein synthesis inhibitor, as confirmed by macromolecular synthesis experiments. Protein synthesis inhibition is thought to occur from binding to the 30S ribosomal subunit. Attachment to the 30S subunit blocks the acceptor site in the mRNA-ribosome complex, which prevents amino acid incorporation into the elongating peptide. Because this interaction is reversible, tetracyclines are generally considered bacteriostatic agents. However, omadacycline has demonstrated in vitro bactericidal activity against Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis. Additionally, modest inhibition of peptidoglycan synthesis may affect protein synthesis.
References
[1] Andrew C. Flick. “Synthetic Approaches to New Drugs Approved during 2018.” Journal of Medicinal Chemistry 63 19 (2020): 10652–10704.
[2] R. Burgos, K. Rodvold. “Omadacycline: a novel aminomethylcycline.” Infection and Drug Resistance 12 1 (2019): 1895–1915.
[3] J. Karlowsky, G. Zhanel, J. Steenbergen. “Microbiology and Preclinical Review of Omadacycline.” Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America 37 1 (2019): S6–S15.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringUpadacitinib is FDA-approved for the treatment of moderate to severe rheumatoid arthritis (RA) that has not responded to first-line therapy.....
Dec 19,2023InhibitorsOmadacycline
389139-89-3You may like
Omadacycline manufacturers
- Omadacycline
-
- $51.00 / 1mg
- 2025-12-10
- CAS:389139-89-3
- Min. Order:
- Purity: 99.92%
- Supply Ability: 10g
- Omadacycline
-

- $0.00 / 10mg
- 2025-09-25
- CAS:389139-89-3
- Min. Order: 10mg
- Purity: 0.98
- Supply Ability: 5g
- Omadacycline
-

- $0.00 / 1kg
- 2025-08-22
- CAS:
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1