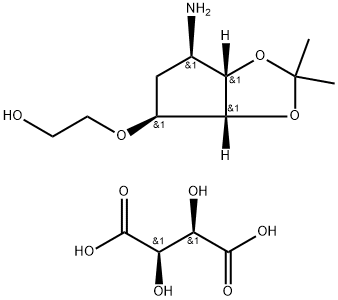
Pharmaceutical Applications of 2-((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yloxy)ethanol L-tataric acid
Nov 28,2025
2-((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yloxy)ethanol L-tataric acid serves as a key intermediate in organic synthesis and pharmaceutical research, specifically for the active pharmaceutical ingredient ticagrelor. Known commercially as Brilinta or Brillinta in some markets, ticagrelor is a platelet aggregation inhibitor used to prevent strokes and myocardial infarctions in patients with acute coronary syndrome.
Synthesis
A stirred solution of 1-acetyladamantane Intermediate (545g) in ethanol (3.81 L) was heated to 35°C. L-tartaric acid (352g) was added and the mixture was stirred at 40-45°C for 1 h. The mixture was cooled to 20°C and the resulting thick slurry stirred for 16 h then filtered. The collected solid was washed with two portions of 2- propanol (300ml, then 500ml), sucked dry then dried in vacuaat 40°C to give the product as white crystals 2-((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yloxy)ethanol L-tataric acid (728 g).
Medical Applications
2-((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yloxy)ethanol L-tataric acid serves as a key synthetic intermediate for the active pharmaceutical ingredient ticagrelor, undergoing sequential chemical transformations to construct this cyclopentyltriazolopyrimidine (CPTP) class oral antiplatelet agent classified under ATC code B01AC24. Originally developed by AstraZeneca with US market approval in July 2011, ticagrelor functions as a non-prodrug that directly binds reversibly to the P2Y12 ADP receptor without requiring hepatic metabolic activation. The pivotal PLATO trial demonstrated that 12-month ticagrelor treatment significantly reduced the composite endpoint of cardiovascular death, myocardial infarction, or stroke by 16% and cardiovascular mortality by 21% compared to clopidogrel in acute coronary syndrome (ACS) patients, without increasing major bleeding risks. Based on these established clinical benefits, 2-((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yloxy)ethanol L-tataric acid has become an indispensable intermediate in manufacturing ticagrelor, which is now guideline-recommended for antiplatelet therapy in ACS management. [1]
Nucleophilic Aromatic Substitution
![Nucleophilic Aromatic Substitution of 2-((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yloxy)ethanol L-tataric acid Nucleophilic Aromatic Substitution of 2-((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yloxy)ethanol L-tataric acid](/NewsImg/2025-11-05/6389797336239305595827950.jpg)
Figure 1: Nucleophilic Aromatic Substitution of 2-((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yloxy)ethanol L-tataric acid
To a stirred solution of triethylamine (326.7 g) in n-butanol (360 mL), 2-((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yloxy)ethanol L-tataric acid (118.8 g) and aminopyrimidine (76.9 g) were added, followed by heating the mixture at 112°C for 32 hours under nitrogen protection. Upon reaction completion, solvent evaporation afforded a dark brown sticky material, which was treated with ethyl acetate (800 mL) and water with stirring for 5 minutes. After layer separation, the aqueous phase was extracted with ethyl acetate (400 mL), and the combined organic layers were washed with water (500 mL × 2), dried, and concentrated to give deep-brown oil. The crude product was treated with a 3:1 (w/w) n-heptane/ethyl acetate solution (1200 mL/400 mL) at 70–80°C for 1 hour, cooled to 10°C, and stirred continuously for 1.5 hours. The resulting precipitate was filtered, washed with n-heptane, and dried at 45°C to yield the title compound as a white solid (117.3 g, 88.2%) with 99.5% purity by HPLC analysis. [2]
Etherification
The reactive hydroxyl group in 2-((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yloxy)ethanol L-tataric acid possesses significant nucleophilic character, enabling its condensation-etherification with chlorosilanes in the presence of basic catalysts such as imidazole to form corresponding silyl ether derivatives.
![Etherification of 2-((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yloxy)ethanol L-tataric acid Etherification of 2-((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yloxy)ethanol L-tataric acid](/NewsImg/2025-11-05/6389797364483235897979950.jpg)
Figure 2: Etherification of 2-((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yloxy)ethanol L-tataric acid
In an meticulously dried reaction flask equipped with standard atmospheric control accessories, the precisely weighed 2-((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yloxy)ethanol L-tataric acid was initially dissolved in rigorously dried dichloromethane under inert atmosphere protection, to which was then gradually introduced sequentially measured imidazole and tert-butyldimethylchlorosilane (TBDMSCl) through controlled dropwise addition over an extended duration. The resulting homogeneous reaction mixture was maintained under continuous magnetic agitation at ambient temperature for approximately 5 hours with real-time reaction monitoring, after which the completed reaction system was subjected to vacuum-assisted filtration through a sintered glass funnel to remove particulate impurities. The collected filtrate was systematically concentrated under reduced pressure using rotary evaporation apparatus until constant weight was achieved, and the resultant viscous residue underwent sophisticated purification through silica gel column chromatography with gradient elution systems to ultimately isolate the target molecular entity with structural integrity. [3]
Reference
[1] Preparation of Novel triazolo pyrimidine compounds as pharmaceuticals, World Intellectual Property Organization, Patent number:WO2001092263 A1.
[2] Wang, Li-hong; et al, An Effective and Convenient Process for the Preparation of Ticagrelor: Optimized by Response Surface Methodology and One-Pot Reaction, Pharmaceutical Chemistry Journal 2019, 52, 813-819.
[3] Huaiyu Yang, Preparation of ((difluorophenyl)cyclopropyl)aza-aromatic fused ring compounds, pharmaceutical compositions as TREK-1 activators and their analgesic activity. Chinese patent, patent number:CN117003756 A.
- Related articles
- Related Qustion
Escin exerts anti-inflammatory/venotonic effects, alleviates neuropathic pain via NF-κB/MAPK pathways, and has antitumor potential.....
Nov 28,2025APIYou may like
- 2-((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yloxy)ethanol L-tataric acid
-
![376608-65-0 2-((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yloxy)ethanol L-tataric acid](/ProductImageEN1/2025-08/Small/6a35618b-5838-4f52-839b-906ba9b1e436.png)
- $10.00 / 1ASSAYS
- 2025-12-19
- CAS:376608-65-0
- Min. Order: 1ASSAYS
- Purity: 99%
- Supply Ability: 1 ton
- 2-[[(3aR,4S,6R,6aS)-6-Aminotetrahydro-2,2-dimethyl-4H-cyclopenta-1,3-dioxol-4-yl]oxy]-ethanol (2R,3R)-2,3-dihydroxybutanedioate
-
![376608-65-0 2-[[(3aR,4S,6R,6aS)-6-Aminotetrahydro-2,2-dimethyl-4H-cyclopenta-1,3-dioxol-4-yl]oxy]-ethanol (2R,3R)-2,3-dihydroxybutanedioate](/ProductImageEN/2022-04/Small/740eeb89-bb51-4633-909d-957acaf956de.jpg)
- $0.00 / 25KG
- 2025-12-18
- CAS:376608-65-0
- Min. Order: 1KG
- Purity: ≥99%
- Supply Ability: 50mt/year
- 2-((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yloxy)ethanol L-tataric acid
-
![376608-65-0 2-((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yloxy)ethanol L-tataric acid](/ProductImageEN1/2024-08/Small/75c4110c-bde1-4eff-979a-e44cf326d376.png)
- $100.00 / 50kg
- 2025-12-16
- CAS:376608-65-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000Ton