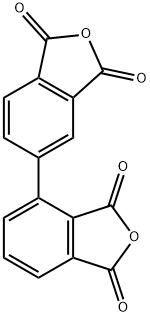
Physical Properties and Polymerization reaction of 2,3,3,4-biphenyl tetracarboxylic dianhydride
Nov 21,2025
2,3,3,4-biphenyl tetracarboxylic dianhydride (a-BPDA, CAS number 36978-41-3), a derivative of biphenyl, has two phthalic anhydrides linked unsymmetrically at 3- and 4-position. The backbone of the polyimides synthesized from 2,3,3,4-biphenyl tetracarboxylic dianhydride is twisted, leading to a suppression of intermolecular charge transfer. Thus, the polyimides are transparent and heat resistant. Diimides synthesized from a-BPDA and cyclohexylamine display long-lived luminescence up to 1.3 seconds after ultraviolet light irradiation.
Physical Properties
2,3,3,4-biphenyl tetracarboxylic dianhydride is used to synthesize covalent organic frameworks(COFs) with low dielectric constant (2.72), low thermal expansion coefficient (62.2 ppm/K) and high breakdown strength (412.8 kV/mm). It is ideal for low signal loss electronic packaging. Lithium battery cathodes of the polyimides-decorated carbon nanotubes (CNTs) show a high capacity of 163 mAh/g at 0.05 A/g and a rate capability of 122 mAh/g at 5 A/g.
Polyimide Material Preparation
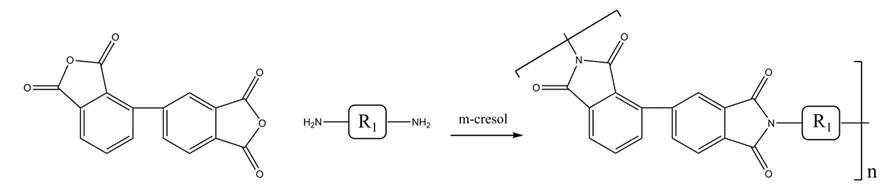
Figure1: Synthesis of the polyimides from 2,3,3,4-biphenyl tetracarboxylic dianhydride
Studies have reported the utility of 2,3,3,4-biphenyl tetracarboxylic dianhydride in polyimide material synthesis, specifically through preparation with aromatic diamines to produce novel polyimides. These advanced materials exhibit integrated characteristics including low colorimetric values, favorable solubility, high thermal emissivity, low solar absorptivity, and superior tensile strength. A series of organosoluble and light-colored polyimides (III) were synthesized from 2,3,3,4-biphenyl tetracarboxylic dianhydride (α-BPDA) and various fluorinated aromatic bis(ether amine)s via a two-step method involving thermal or chemical imidization of poly(amic acid) precursors. The III series derived from 2,3,3,4-biphenyl tetracarboxylic dianhydride exhibited inherent viscosities of 0.74–1.01 dL/g and demonstrated excellent solubility in various organic solvents, including polar amide solvents, ether-type solvents, and chlorinated solvents. The resulting polyimide films showed high optical transparency with low color intensity, featuring an ultraviolet-visible absorption edge of 369–382 nm and low b* values (yellowness index) of 5.0–11.7. These polymers displayed glass transition temperatures of 244–319°C, higher than those of isomeric polyimide V series, and compared with non-fluorinated polyimide IV series, the III series exhibited lighter color, lower dielectric constants, reduced moisture absorption, along with satisfactory tensile properties and outstanding thermal stability. [1-2]
Polymerization reaction
The polyimides were synthesized through equimolar polyaddition between 2,3,3,4-biphenyl tetracarboxylic dianhydride (a-BPDA) and diamine monomers via a one-step method in m-cresol, as illustrated in Scheme 1. In a representative procedure, 2,3,3,4-biphenyl tetracarboxylic dianhydride (a-BPDA) (2.1185 g, 7.20 mmol), TFMB (2.3071 g, 7.20 mmol), isoquinoline (0.45 g, 3.5 mmol), and m-cresol (25 g) were charged into a 100 mL three-necked flask equipped with a mechanical stirrer, condenser, and nitrogen inlet/outlet. The mixture was first stirred at 160°C for 1 hour to achieve complete dissolution of reactants, then heated to 200°C and maintained at this temperature for 10 hours. The resulting viscous solution was cooled and precipitated into an excess of ethanol. The fibrous product was subsequently washed multiple times with ethanol and dried at 120°C under vacuum for 24 hours to yield the polyimide a-BPDA/TFMB. [3]
Synthetic Process
Method 1
A facile method for the synthesis of 2,3,3,4-biphenyl tetracarboxylic dianhydride (a-BPDA) was reported, which comprises the steps of the dehalogenative coupling of dimethyl 4-chlorophthalate(4-DMCP) and dimethyl 3-chlorophthalate(3-DMCP) catalyzed by low-cost (PhP)NiCl, the hydrolysis of tetra-ester and the dehydration of tetra-acid. In contrast to the conventional methods, this method has the advantage of low cost, convenient manipulation, available condition, high purity and good overall yield. Moreover, the single crystal structure of a-BPDA was analyzed by X-ray diffraction method. The X-ray data suggest that 2,3,3,4-biphenyl tetracarboxylic dianhydride is a rigid, non-coplanar and non-linear structure. It contains three crystallographically independent molecules, in which the dihedral angles of the two linked phenyl rings are 44.75(4)°, 46.37(3)° and 42.32(3)°, respectively. The title molecule is governed by a stronger intermolecular interaction in contrast to van der Waals interaction because of the special positions of anhydride groups. [3]
Method 2
2,3,3′,4′-biphenyltetracarboxylic acid is heat-dehydrated in a molten state at a temperature not lower than 200° C in a flow of an inert gas in a reactor 10 having at least one reaction vessel by stirring the molten material to produce 2,3,3′,4′-biphenyltetracarboxylic dianhydride. Thus obtained 2,3,3,4-biphenyl tetracarboxylic dianhydride in the molten state is subsequently cooled and solidified in an inert gas or dry air, or cooled and solidified in the ambient air at a temperature of 40° C or lower or 100° C or higher. [4]
Reference
[1] Hergenrother P M , Smith J G , Connell J W ,et al.Polyimides from 2,3,3′,4′-biphenyltetracarboxylic dianhydride and aromatic diamines[J]. Polymer: The International Journal for the Science and Technology of Polymers, 2002, 19:43.
[2] Yang C P , Su Y Y .Colorless polyimides from 2,3,3′,4′-biphenyltetracarboxylic dianhydride (α-BPDA) and various aromatic bis(ether amine)s bearing pendent trifluoromethyl groups[J].Polymer, 2005, 46: 5797-5807.
[3] Song, G. "Colorless, heat resistant polyimide films derived from 2, 3, 3′, 4′-biphenyltetracarboxylic dianhydride." IOP Conference Series: Materials Science and Engineering. 2020, 733.
[4] Tatsushi Nakayama,Takeshi Matsuzaki,Kenichiro Sasaki.Process for production of 2,3,3′,4′-biphenyltetracarboxylic dianhydride:US11208823[P].
- Related articles
- Related Qustion
Pentaerythritol Tetra(3-mercaptopropionate) is derived from pentaerythritol fully esterified with four equivalents of 3-mercaptopropionic acid.....
Nov 21,2025APIOverview of LiDFP’s roles, mechanisms, and performance benefits across lithium-ion and lithium-metal battery systems.....
Nov 21,2025API2,3,3,4-biphenyl tetracarboxylic dianhydride
36978-41-3You may like
2,3,3,4-biphenyl tetracarboxylic dianhydride manufacturers
- 2,3,3',4'-Biphenyltetracarboxylic dianhydride
-

- $0.00 / 25KG
- 2025-12-12
- CAS:36978-41-3
- Min. Order: 1KG
- Purity: ≥99%
- Supply Ability: 200mt/year
- 2,3,3',4'-BiphenyLtetracarboxylic (α-BPDA)
-
- $0.00 / 1kg
- 2025-10-27
- CAS:36978-41-3
- Min. Order: 1kg
- Purity: ≥99.9%
- Supply Ability: 1MT
- 2,3,3',4'-Biphenyl tetracarboxylic dianhydride
-
- $122.00 / 1KG
- 2025-09-25
- CAS:36978-41-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available