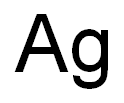Silver-Health Hazards and Toxicity
Sep 6,2019
Silver’s modern chemical symbol (Ag) is derived from its Latin word argentum, which means silver. The word “silver” is from the Anglo-Saxon world “siolfor.” Ancients who first refined and worked with silver used the symbol of a crescent moon to represent the metal.Silver is located in group 11 (IB) of period 5, between copper (Cu) above it in period 4 andgold (Au) below it in period 6.

Toxicity Data
PEL (OSHA) 0.01 mg/m3
TLV-TWA (ACGIH) 0.1 mg/m3 (silver metal)
TLV-TWA (ACGIH) 0.01 mg/m3 (soluble silver compounds, as Ag)
Major Hazards
Exposure to silver metal or soluble silver compounds can cause discoloration or bluegray darkening of the eyes, nose, throat, and skin.
Fire Hazard
Silver and most soluble silver compounds are not combustible. However, silver nitrate and certain other silver compounds are oxidizers and can increase the flammability of combustible materials. Silver acetylide, azide, fulminate, oxalate mixtures, styphnate, tartarate mixtures, and tetrazene are all explosives and must be handled as such.
Toxicity
The acute toxicity of silver metal is low. The acute toxicity of soluble silver compounds depends on the counterion and must be evaluated case by case. For example, silver nitrate is strongly corrosive and can cause burns and permanent damage to the eyes and skin.
Chronic exposure to silver or silver salts can cause a local or generalized darkening of the mucous membranes, skin, and eyes known as argyria. The other chronic effects of silver compounds must be evaluated individually.
Flammability and Explosibility
Silver and most soluble silver compounds are not combustible. However, silver nitrate and certain other silver compounds are oxidizers and can increase the flammability of combustible materials.
Silver acetylide, azide, fulminate, oxalate mixtures, styphnate, tartarate mixtures, and tetrazene are all explosives and must be handled as such.
Reactivity and Incompatibility
Contact of metallic silver and silver compounds with acetylene may cause formation of silver acetylide, which is a shock-sensitive explosive. Contact with ammonia may cause formation of compounds that are explosive when dry. Contact with strong hydrogen peroxide solutions causes violent decomposition with the formation of oxygen gas.
Many silver compounds are light sensitive, and many have significant reactivities or incompatibilities, which should be evaluated before use.
Storage and Handling
Silver and silver compounds should be handled in the laboratory using the "basic prudent practices". Individual silver compounds should be evaluated on a case-by-case basis to determine whether additional handling procedures for high toxicity or reactivity and explosibility are appropriate. Most silver compounds should be protected from light during storage or while in use.
Accidents
In the event of skin contact, immediately wash with soap and water and remove contaminated clothing. In case of eye contact, promptly wash with copious amounts of water for 15 min (lifting upper and lower lids occasionally) and obtain medical attention. If silver or silver compounds are ingested, obtain medical attention immediately. If large amounts of silver dust or silver compounds are inhaled, move the person to fresh air and seek medical attention at once.
In the event of a spill, sweep up the silver or silver compounds or soak up with a nonreactive absorbent material or spill pillow, place in an appropriate container, and dispose of properly. Respiratory protection may be necessary in the event of a large spill or release in a confined area.
Disposal
Excess silver, silver compounds, and waste material containing these substances should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines. Collection for silver recovery should be considered.
- Related articles
- Related Qustion
- 10 Interesting Facts About Silver Mar 5, 2024
Silver (Ag), atomic number 47, is a rare metal resource with dull-white color. In nature, most of the silver resources are associated with Lead-Zinc ores, except for a small portion in the form of simple substance or silver minerals.
- What is the charge of silver? Feb 18, 2024
Silver typically has a +1 charge when involved in an ionic bond. Although silver can form both +1 and +2 cations, the +2 is so rare that we usually name Ag + as silver ion, not silver(I) ion.
- Flame test and chemical principles of silver Jan 25, 2024
Silver do not produce a characteristic flame test color. There are several possible explanations for this.
The acute toxicity of pyridine is low. Inhalation causes irritation of the respiratory system and may affect the central nervous system, causing headache, nausea, vomiting, dizziness, and nervousness.....
Sep 6,2019Flavors and fragrancesBenzene is a colorless, volatile, highly flammable liquid that is used extensively in the chemical industry and received wide interest in the early days of organic chemistry.Because of its structure, benzene is a very stable organic compoun....
Sep 6,2019Organic ChemistrySilver
7440-22-4You may like
- Silver
-

- 2025-12-05
- CAS:7440-22-4
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Nanometer silver solution
-

- $410.00 / 1KG
- 2025-12-03
- CAS:7440-22-4
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 1-20mt
- Silver
-

- $685.00 / 1ton
- 2025-09-26
- CAS:7440-22-4
- Min. Order: 1ton
- Purity: 99%
- Supply Ability: 900ton